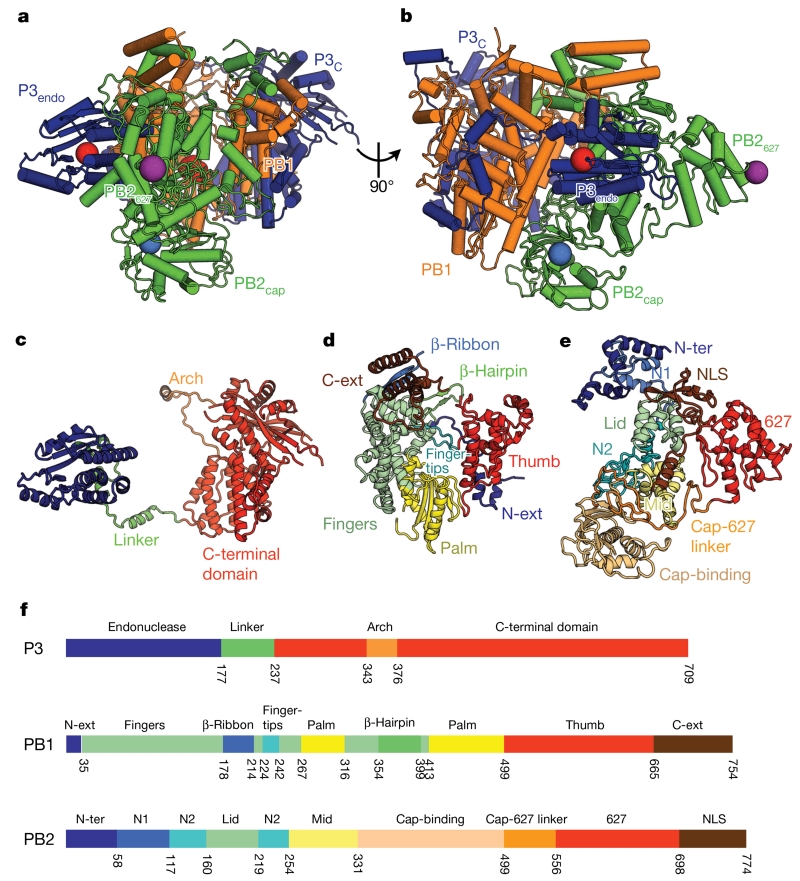
Figure 1. Structure of FluPolC.
a, b, Two views of the structure of the FluPolC heterotrimer, coloured according to subunit (PB1, orange; PB2, green; P3, blue). The cap-binding pocket and endonuclease active site are shown as blue and red spheres, respectively. In a, the PB1 catalytic aspartates, residues 446 and 447, are also highlighted red. The position of PB2 residue 649 (equivalent to PB2 residue 627 in FluPolA) is marked by a purple sphere. c–e, Structures of FluPolC subunits P3 (c), PB1 (d) and PB2 (e), coloured and labelled by domain. f, Domain maps of each FluPolC subunit.