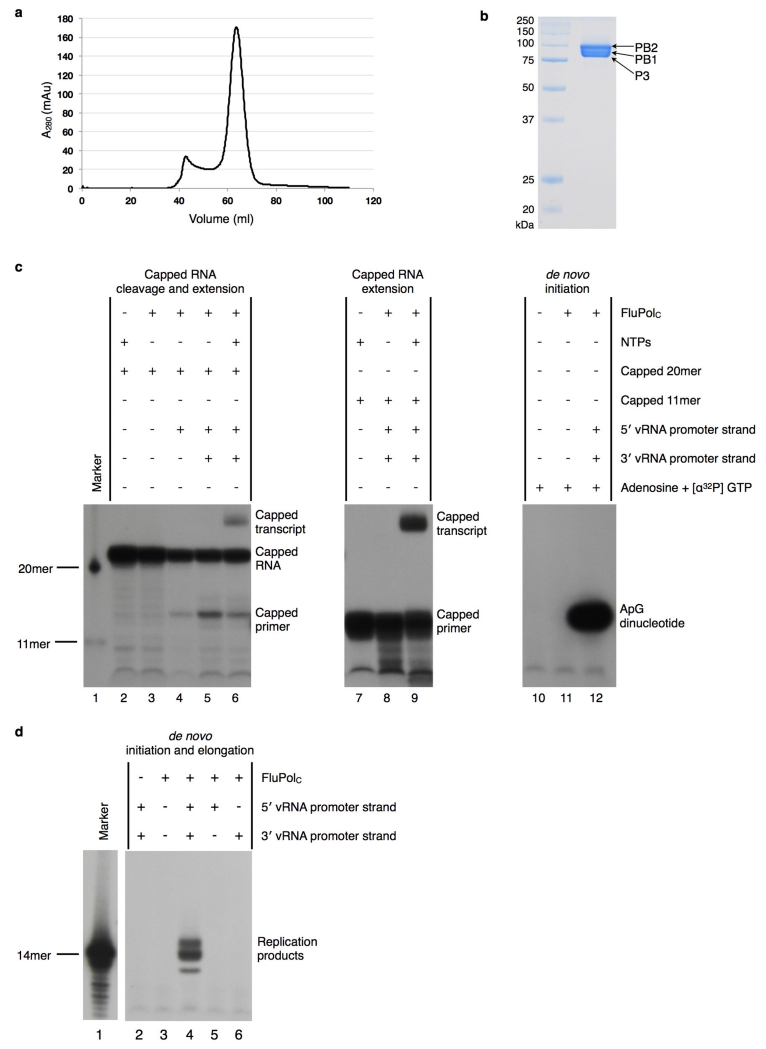
Extended Data Figure 1. Purification and characterization of FluPolC.
a, Elution profile of FluPolC, after affinity purification over IgG-sepharose, from a size-exclusion chromatography column. Eluted protein was detected by measuring the absorbance at 280 nm. b, Fractions corresponding to the major peak eluting from the size-exclusion chromatography column were mixed and analysed by SDS–PAGE on a 15% polyacrylamide gel, alongside the indicated molecular mass markers. Protein was visualized by Coomassie blue staining (PB1, 86.0 kDa; PB2, 87.2 kDa; P3, 81.9 kDa). c, Transcription and replication initiation assays. Lanes 2–6 test for transcription initiation. With the addition of vRNA promoter only (lanes 4 and 5), FluPolC can cleave a capped and radiolabelled 20-nucleotide RNA, demonstrating promoter-dependent endonuclease activity. Lane 6 shows that with the addition of NTPs, this capped primer can be extended to produce a capped transcript, thus demonstrating transcription initiation activity. This result is confirmed by lanes 7–9, which test for extension of a capped and radiolabelled 11-nucleotide RNA primer. Extension only takes place when the polymerase is supplied with NTPs and promoter RNA (lane 9). Lanes 10–12 assay for replication initiation. Lane 12 shows that FluPolC (400 ng per reaction) is able to synthesize ApG dinucleotide in a primer-independent manner. This demonstrates de novo replication initiation activity. Uncapped 20-nucleotide and 11-nucleotide primers are used as size markers in lane 1. The slow migration of the ApG dinucleotide compared to the markers is due to the lack of phosphate groups on the 5′ end of this product35. d, De novo initiation and elongation assay. FluPolC (800 ng) was incubated for 3 h with NTPs, [α-32P]GTP and 5′ or 3′ vRNA promoter strands, as indicated. In the presence of both promoter strands (lane 4), FluPolC is able to produce a full-length copy of the template (14 nucleotides, corresponding to the major band), demonstrating de novo replication initiation and elongation activity. The minor slower and faster bands may correspond to non-templated extension and premature termination products, respectively.