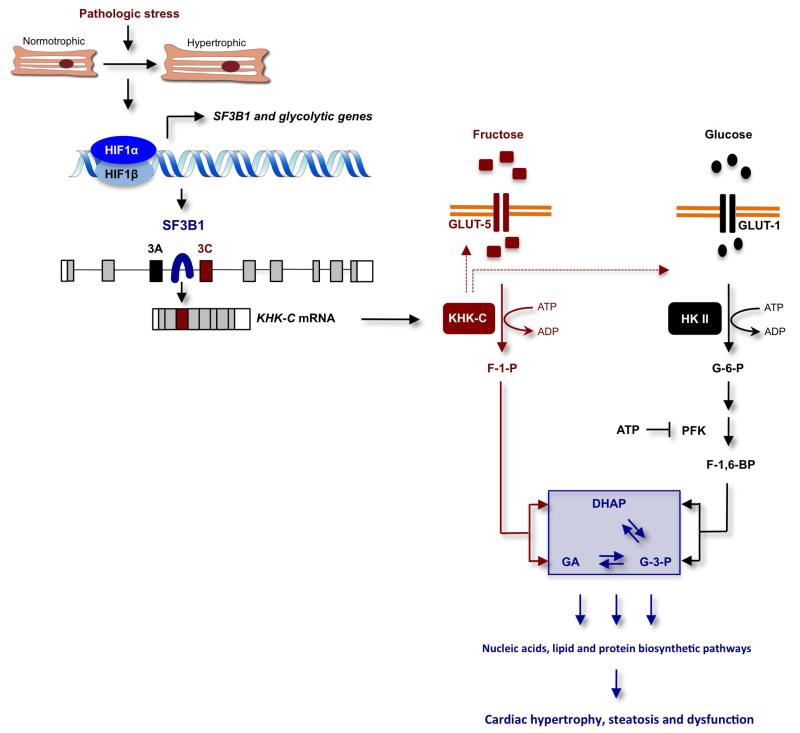
Extended Data Figure 7. Model explaining how HIF1α activation of SF3B1-dependent splicing of KHK regulates fructose and glucose metabolism to promote cardiac hypertrophy in response to pathologic stress.
In this model, pathologic stress leads to increased expression of HIF1α and HIF1α-dependent activation of genes encoding glycolytic enzymes and the splicing factor SF3B1. SF3B1, in turn, assembles at the branch-point sequence upstream of exon 3C of KHK pre-mRNA leading to an inclusion of this exon and KHK-C protein production. This shift in isoform expression from KHK-A to KHK-C in response to HIF1α–SF3B1 pathway activation drives KHK-C-dependent fructose uptake via stimulation of GLUT5 expression, the conversion of fructose to fructose-1-phosphate (F1P) and contributes simultaneously to the activation of glucose uptake and metabolism through a yet-to-be-determined mechanism. The model holds that the unrestrained conversion of fructose to F1P by KHK-C limits ATP levels, thereby alleviating potential allosteric inhibition of phosphofructokinase (PFK) by ATP to maintain a high glycolytic flux. F1P is further metabolized to dehydroxyacetone phosphate (DHAP) and glyceraldehyde (GA). While DHAP serves as a precursor for glycerol synthesis, GA can be further converted to glyceraldehyde-3-phosphate (G3P), a key glycolytic intermediate. G3P can be channelled into the non-oxidative pentose phosphate pathway (PPP) supporting nucleic and amino acid biosynthesis. This metabolic constellation, created by the activation of the HIF1α–SF3B1–KHK-C axis, increases macromolecular biosynthetic capacity essential for hypertrophic growth, steatosis and cardiac dysfunction. HKII and F-1,6-BP denotes hexokinase II and fructose-1,6-bisphosphate, respectively.