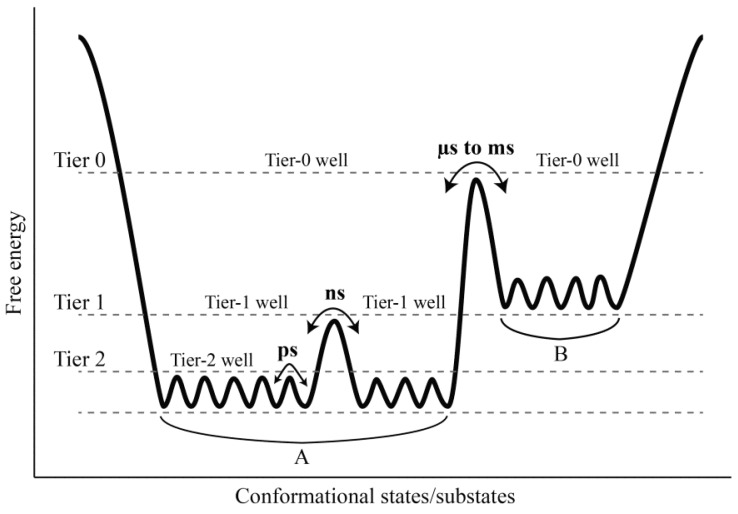
Figure 7.
A proposed FEL model to explain the effect of the solvent mobility on protein dynamics. This model is represented as a hierarchical organization of free energy wells (i.e., the smallest tier-2 wells are within the relatively large tier-1 wells, and the tier-1 wells are within the largest tier-0 wells), which dictates the hierarchical dynamics of the protein (i.e., different structural components feature different amplitude and timescale of the fluctuations). The tier-2 and tier-1 substates and tier-0 states (A and B) are located within respective free energy wells. The tier-2, tier-1, and tier-0 dynamics, which are defined as conformational interconversion between respective substates/states, involve the side chain rotations on ps timescale, loop motions on ns timescale, and collective motions of the entire structure on timescale of μs to ms, respectively. The tire-0 dynamics are a result of the accumulation of the tier-1 and tier-2 dynamics. By exacerbating the competitive interactions between the protein and solvent and between atoms within the protein, the solvent mobility plays its role in facilitating the cascade amplification of microscopic motions of atoms and atomic groups into the global collective motions of the protein (for details, see the text).