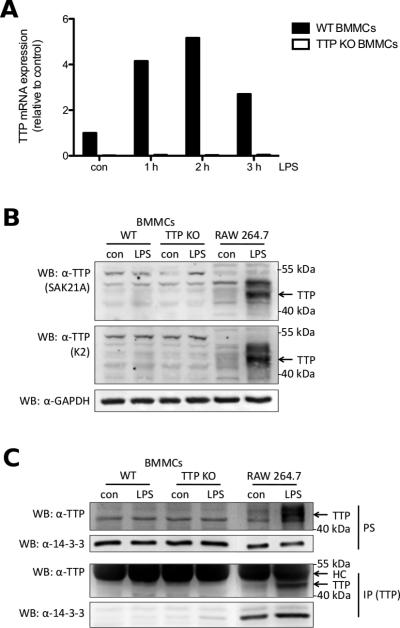
Fig. 5.
Despite TTP gene expression no TTP protein is detectable in WT BMMCs. (A) WT (■) and TTP-deficient (KO) BMMCs (□) were left untreated (con) or stimulated with 3 μg/ml LPS for the indicated times. TTP mRNA was analyzed by RT-qPCR. Data is representative of three independent experiments using seperate cell cultures (see Suppl. Fig. 3). (B) WT BMMCs, TTP-deficient BMMCs, and RAW 264.7 macrophages were left untreated (con) or stimulated with LPS for 4 h (3 μg/ml for BMMCs, 0.1 μg/ml for RAW macrophages). Postnuclear supernatants (PS) (40 μg protein for BMMCs, 20 μg protein for RAW macrophages) were analyzed by immunoblotting with antisera raised against the C-terminus of TTP (SAK21A [23] top panel, K2 [24] middle panel) and an antibody against GAPDH (bottom panel). (C) WT, TTP-deficient BMMCs and RAW 264.7 macrophages were left untreated (con) or stimulated with LPS for 3 h (3 μg/ml for BMMCs, 0.1 μg/ml for RAW macrophages). Pellets were lysed and PS were subjected to anti-TTP immunoprecipitation. Precipitates (IP, third and fourth panel from top) as well as PS (first and second panel from top) were analyzed by anti-TTP (first and third panel from top) and anti-14-3-3 (second and fourth panel from top) immunoblotting. HC, heavy chain