Figure 1. TrkB receptors interact and co-localize with Slitrk5.
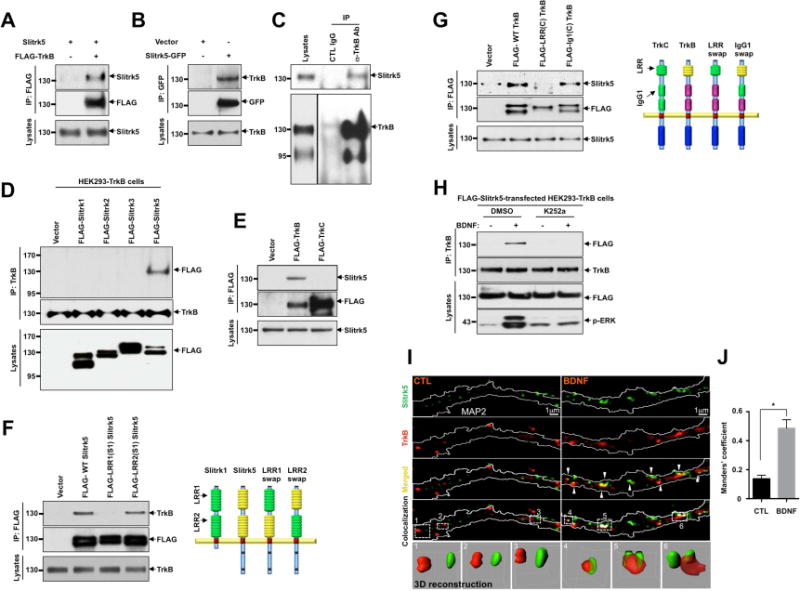
(A) Interaction between TrkB receptors and Slitrk5 was assessed in HEK293T overexpressing cDNAs encoding FLAG-TrkB, Slitrk5, and empty vector. Cell lysates were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-Slitrk5 antibodies. (B) Interaction between TrkB receptors and Slitrk5 was assessed in HEK293-TrkB cells. Lysates from Slitrk5-GFP or empty vector transfected HEK293-TrkB cells were immunoprecipitated with anti-GFP antibodies and immunoblotted with anti-TrkB antibody. (C) Endogenous association of Slitrk5 and TrkB. Mouse whole-brain lysates (2 months old) were subjected to immunoprecipitation with anti-TrkB antibody (Millipore) or control IgG. The immune protein complex was then eluted, and TrkB and Slitrk5 were detected by immunoblotting. (D) and (E) Interaction between TrkB and Slitrk5 is specific. (D) Interaction between TrkB and Slitrk family members. FLAG-tagged Slitrk family isotypes were expressed in HEK293-TrkB cells. Cell lysates were immunoprecipitated with anti-TrkB (Millipore) antibodies and immunoblotted with anti-FLAG (M2) antibodies (E) Dissociated E16 mouse cortical neurons were electroporated (Amaxa) with FLAG-TrkB or FLAG-TrkC. At DIV6, cell lysates were precipitated with anti-FLAG (M2) antibodies and immunoblotted with anti-Slitrk5 antibodies. (F) First LRR domain of Slitrk5 mediates TrkB binding. HEK293-TrkB cells were transfected with FLAG-tagged WT Slitrk5, chimeric FLAG-LRR1-domain-swapped (FLAG-LRR1(S1) Slitrk5) and LRR2-domain-swapped Slitrk5 (FLAG-LRR2(S1) Slitrk5) or empty vector. Cell lysates were precipitated with anti-FLAG (M2) antibodies and immunoblotted with anti-TrkB (Millipore) antibodies. Schematic representation of chimeric Slitrk5 mutants shown on the right. (G) LRR domain of TrkB mediates Slitrk5 binding. Dissociated E16 mouse cortical neurons were transfected with FLAG-tagged WT TrkB, LRR domain-swapped TrkB (FLAG-LRR(C) TrkB), and IgG1 domain-swapped TrkB (FLAG-IgG1(C) TrkB) or empty vector. At DIV6, cell lysates were immunoprecipitated with anti-FLAG antibodies and immunoblotted with anti-Slitrk5. Schematic representation of chimeric TrkB mutants shown on the right. (H) BDNF-dependent and TrkB kinase activity-dependent binding of TrkB and Slitrk5. After transfection of FLAG-tagged Slitrk5 into HEK293-TrkB cells, cells were pretreated with DMSO or K252a for 30 min to inhibit kinase activity of TrkB after overnight serum starvation. Cells were treated with or without BDNF, and their binding was examined by immunoprecipitation with anti-TrkB antibodies followed by immunoblotting with anti-FLAG antibodies. (I) TrkB receptors co-localize with Slitrk5 in a BDNF-dependent manner. DIV6 striatal neurons were treated with or without BDNF (25ng/ml) for 30 min after incubating with anti-TrkB antibody to specifically label surface TrkB. Endogenous Slitrk5 and MAP2 were visualized with specific antibodies after fixation and permeabilization. Super resolution images were acquired using a Nikon N-SIM structured illumination microscope. The co-localization of TrkB and Slitrk5 was presented using co-localization highlighter (ImageJ) and 3D reconstruction (IMARIS) to show more convincing co-localization signals. (J) Histogram showing mean Manders’ coefficients for co-localization of TrkB and Slitrk5 (n=20 for each condition). High Manders’ coefficients indicate better co-localization of TrkB with Slitrk5. Results are means ± SEM from 3 independent experiments. 20–30 neurons were analyzed per condition per experiment. *P<0.05 significantly different from control condition (Student’s t test).
