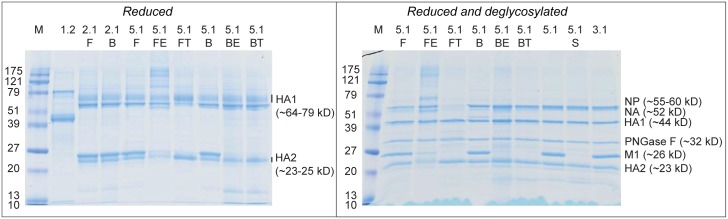
Fig 2. SDS PAGE of reduced and reduced plus de-glycosylated samples as a fingerprint of principle proteins present.
Lanes M were loaded with marker proteins, with the corresponding molecular weight presented to the left. The fraction sample identity (Fig 1) is noted above the lane. Left gel: 1.2 before ZUC, 2.1F after ZUC in phosphate, 2.1B after ZUC in citrate, followed by the six bulks (5.1F, 5.1FE, 5.1FT, 5.1B, 5.1BE and 5.1BT); the migration distance of heavily glycosylated HA proteins varies, causing diffuse bands. In such a case the HA1 band range (~64–79 kD) may be difficult to discriminate from the Nucleoprotein band (~55–66 kD) and the HA2 band range (~23–25 kD) may cover the location of M1 band (~26 kD) as reported by Harvey [22]. After de-glycosylation the HA bands are more distinct and migration distance has increased (right gel, bulks 5.1F, 5.1FE, 5.1FT, 5.1B, 5.1BE and 5.1BT). NP and M1 protein bands have not changed position due to the applied de-glycosylation. In the lanes to the right of the right gel, for comparison products prepared at Intravacc site were applied: 5.1 is WIV BPL inactivated bulk, 5.1S is BPL inactivated Triton split bulk and 3.1 is BPL inactivated influenza before splitting with Triton