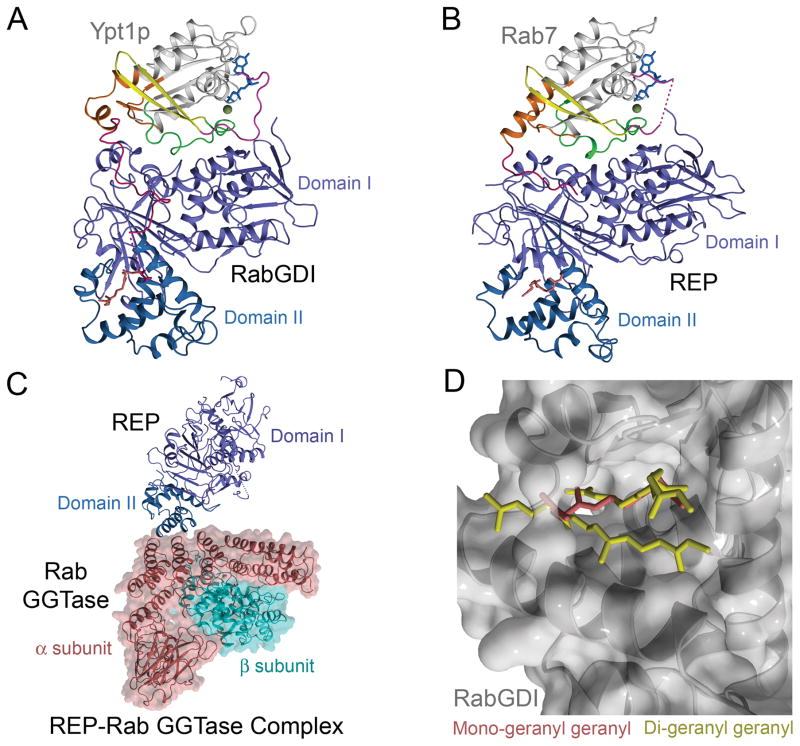
Figure 2. Structures of RabGDI and REP complexes with prenylated Rab GTPases or RabGGTase illuminate the structural mechanisms for membrane targeting.
A) Structure of RabGDI in complex with mono-geranyl geranylated Ypt1p. B) Structure of REP in complex with mono-geranyl geranylated Rab7. Note overall similarity to the RabGDI-Ypt1p complex with respect to the protein-protein interface and location of the prenyl binding pocket. C) Structure of REP in complex with RabGGTase. Docking of Domain II with the α subunit of RabGGTase orients the Rab binding platform in Domain I of REP such that the flexible C-terminus of bound Rab GTPases can readily extend to the active site in the β subunit. D) Comparison of mono- and di-geranyl geranyl binding to domain II of RabGDI in the complexes with prenylated Ypt1p.