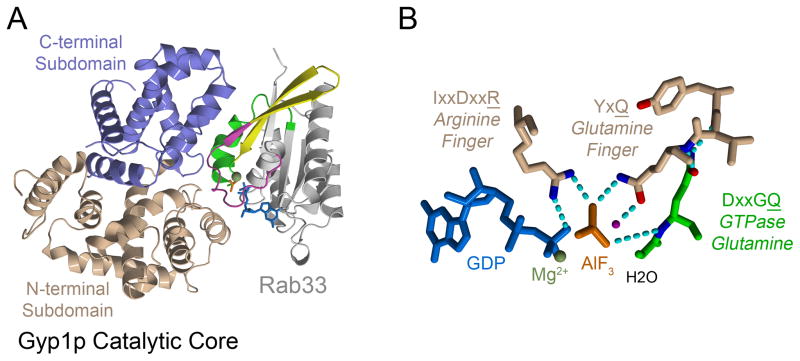
Figure 4. The structure of the Gyp1p TBC domain in a ternary complex with GDP-bound Rab33 and the transitions state mimic AlF3 suggests that TBC domains are dual finger GAPs.
A) Overall structure of the Gyp1p TBC domain-Rab33-GDP-AlF3 ternary complex. B) The N-terminal subdomain of the Gyp1p TBC domain stabilizes the AlF3 transition state mimetic complex by supplying two catalytic residues in trans, an ‘arginine finger’ from the IxxDxxR motif and a ‘glutamine finger’ from the YxQ motif. The DxxGQ glutamine in Rab33 interacts with the Gyp1p backbone but does not directly participate in stabilization of the AlF3 transition state mimetic.