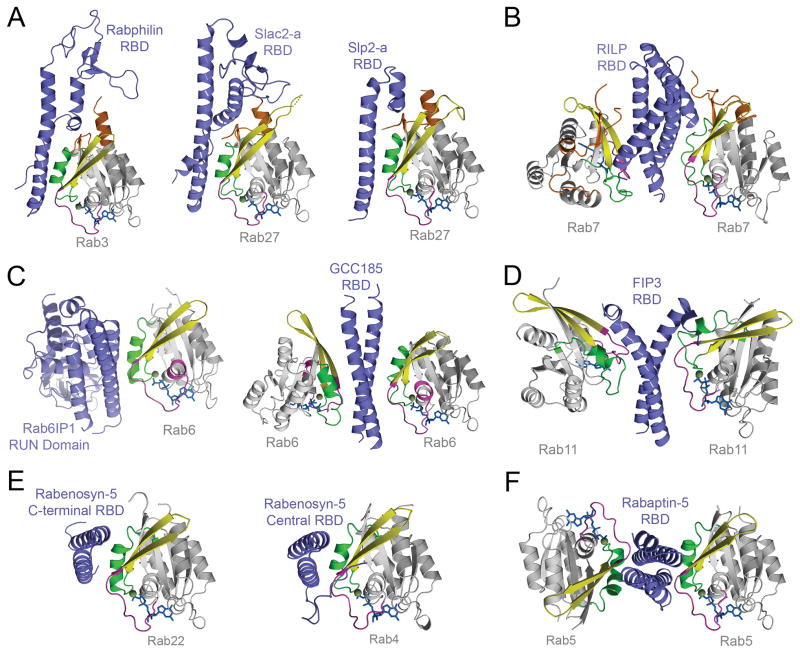
Figure 5. Structures of effector RBDs in complex with active Rab GTPases illustrate similarity and diversity in Rab binding modalities.
A) The structurally related RBDs of Rabphilin-3A, Slac2-a, and Slp2-a form an extended interface with Rab3 or Rab27 that includes the switch/interswitch regions and all three CDRs. B) Interleaved helical hairpins in the RILP RBD support 2:2 binding of a dyad symmetric coiled coil-like dimer to the switch/interswitch regions and N-terminal CDR of Rab7. C) The RUN domain of Rab6IP1 uses a coiled-coil like arrangement of adjacent helices to engage the switch/interswitch regions of Rab6 in a manner analogous to the dyad symmetric coiled coil RBD of GCC185, which supports 2:2 binding. D) The dyad symmetric coiled coil RBD of FIP3 flares apart at the C-terminus to facilitate 2:2 binding to the switch/interswitch regions of Rab11. FIP3 binding induces a large change in the active conformation of switch II. E) The central and C-terminal RBDs of Rabenosyn-5 combine a conserved helical hairpin core with variable N-terminal extensions to engage the switch/interswitch regions of Rab22 or Rab4, respectively. F) The C-terminal dyad symmetric coiled coil RBD of Rabaptin-5 supports 2:2 binding to the switch/interswitch regions of Rab5 with an orientation similar to the asymmetric antiparallel helical hairpins in Rabenosyn-5.