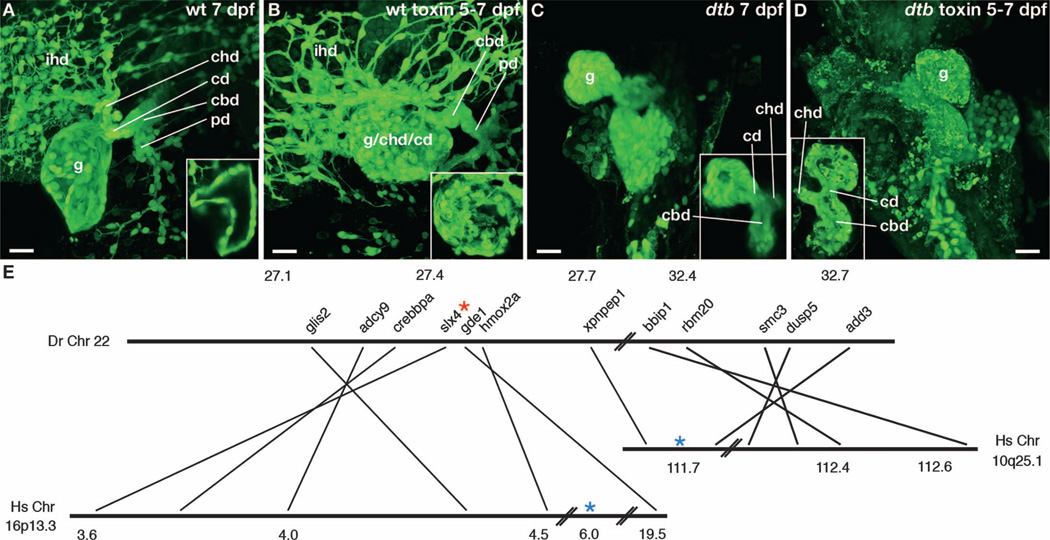
Fig. 4. Zebrafish ductbend sensitization.
| (A to D) Confocal projections through the liver and extrahepatic biliary system of control and toxin-treated wt and ductbend (dtb) larvae. Insets show thin sections through the gallbladder (A and B) or gallbladder and extrahepatic duct (C and D). Compared to the control larvae, extrahepatic BA (A) is evident in the treated wt larva (B), because the three principal components of the extrahepatic system (gallbladder, common bile duct, and cystic duct) distal to the common hepatic duct (chd) cannot be identified. (C) The control ductbend larva lacks intrahepatic bile ducts and has a hypoplastic gallbladder. (D) Toxin-induced damage to the extrahepatic system is more pronounced in the treated ductbend larva compared with wt (C). wt and ductbend larvae were treated with biliatresone (1 µg/ml) from 5 to 7 dpf. (E) Simplified genetic synteny map of the ductbend locus indicating the position of genes relative to their human orthologs at the BA susceptibility loci (16p13.3 and 10q25.1). Numbers refer to chromosomal location in Mb. Blue asterisks, positions of SNPs in BA susceptibility loci; red asterisk, position of SNP 27434434, ~1 centimorgan (cM) (0.6 Mb) from the ductbend locus. ihd, intrahepatic bile ducts; g, gallbladder; cbd, common bile duct; cd, cystic duct; chd, common hepatic duct; pd, pancreatic duct. Scale bars, 20 µm.