Abstract
Ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole) is an antineoplastic agent that intercalates into DNA and alters topoisomerase II activity. Unfortunately, this compound displays a number of adverse properties. Therefore, to investigate new ellipticine-based compounds for their potential as topoisomerase II-targeted drugs, we synthesized two novel derivatives, N-methyl-5-demethyl ellipticine (ET-1) and 2-methyl-N-methyl-5-demethyl ellipticinium iodide (ET-2). As determined by DNA decatenation and cleavage assays, ET-1 and ET-2 act as catalytic inhibitors of human topoisomerase IIα and are both more potent than the parent compound. Neither compound impairs the ability of the type II enzyme to bind its DNA substrate. Finally, the potency of ET-1 and ET-2 as catalytic inhibitors of topoisomerase IIα appears to be related to their ability to intercalate into the double helix.
Keywords: Ellipticine derivatives, DNA topoisomerase IIα, Anticancer drugs, Catalytic inhibitor, DNA cleavage, DNA intercalation
Graphical Abstract
Type II topoisomerases have been important enzyme targets for medicinal and chemotherapeutic agents since the identification of amsacrine as a topoisomerase II-targeted anticancer drug in 1984.1 Since that time, an increasing number of naturally occurring and synthetic compounds of pharmacological importance have been reported to exert their activities by interfering with DNA topoisomerase II function.2–12 Although lower eukaryotic species encode only one type II topoisomerase, vertebrates encode two closely related isoforms of the enzyme, topoisomerase IIα and IIβ.13–18 These enzymes play essential roles in a number of genetic processes, including DNA replication, transcription, and recombination, as well as chromosome segregation.13–18 Type II topoisomerases resolve the problems associated with the topological constraints of the genetic material (i.e., DNA under- or overwinding, knotting, and tangling) by transiently cleaving both strands of the double helix.13–18
Topoisomerase II-targeted agents act in one of two manners.14,15,19–24 Topoisomerase II poisons kill cells by increasing levels of covalent topoisomerase II-cleaved DNA complexes. All of the clinically relevant topoisomerase II poisons examined to date do so by interfering with the ability of the enzyme to religate cleaved DNA molecules.14,15,19,20,22–24 Alternatively, topoisomerase II catalytic inhibitors act by robbing the cell of the essential catalytic functions of the type II enzymes.19–21,23 Catalytic inhibitors have been shown to act at a variety of steps of the topoisomerase II catalytic cycle, including DNA cleavage.19–21,23
Ellipticine (5,11-dimethyl-6H-pyrido[4,3-b]carbazole), a natural product first isolated from the Australian evergreen tree, is an antineoplastic agent that intercalates into DNA and alters topoisomerase II activity.1,25–32 The compound is a mild poison against topoisomerase II from Drosophila and Saccharomyces cerevisiae.29,30 However, most studies report that ellipticine induces little, if any, DNA cleavage mediated by mammalian type II topoisomerases and inhibits enzyme activity at higher concentrations.1,27–29 In contrast to the parent compound, several ellipticine derivatives are potent topoisomerase II poisons in mammalian systems and display anticancer activity against human breast cancer and other solid tumors.26,32,33 Unfortunately, these compounds induce a number of adverse effects such as dry mouth, weight loss, hemolysis, and renal toxicity. Moreover, the parent compound, ellipticine, displays poor water solubility and target specificity, and drug resistance has been observed upon prolonged administration.27,34–36
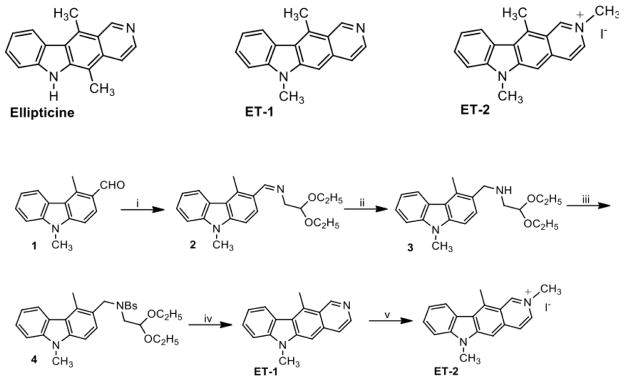
The activities of hydroxylated and N-alkylated ellipticine analogs have been described previously.32,33 However, the properties of C5 demethylated compounds have not been analyzed. Thus, in an effort to investigate new ellipticine-based compounds for their potential as topoisomerase II-targeted drugs, we synthesized two novel derivatives, N-methyl-5-demethyl ellipticine (ET-1) and 2-methyl-N-methyl-5-demethyl ellipticinium iodide (ET-2). Ellipticine derivatives were synthesized via a novel pathway shown in Fig. 1. The detailed syntheses and physical and chemical characterizations of ET-1 and ET-2 are described in the accompanying Supplementary Data.
Figure 1.
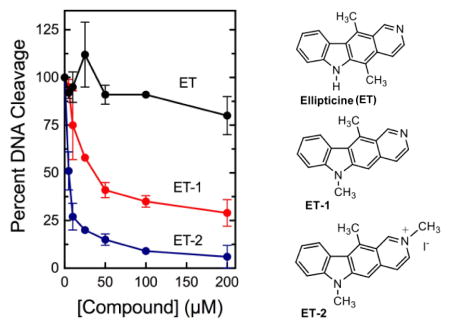
Structures and synthetic pathway of the compounds utilized in this study. Top) The structures of ellipticine, N-methyl-5-demethyl ellipticine (ET-1), and 2-methyl-N-methyl-5-demethyl ellipticinium iodide (ET-2) are shown. Bottom) The approach used to synthesize ellipticine derivatives ET-1 and ET-2 is shown. Reagents and Conditions: i) aminoacetaldehyde diethyl acetal, 100 °C, stirred, 4 h.; ii) NaBH4, methanol, RT, stirred, 2 h.; iii) N(C2H5)3, chloroform, benzenesulfonyl chloride, stirred, RT, 18 h.; iv) 6 N HCl, dioxane, N2, reflux, 2 h.; v) CH3I, DMF, stirred, RT, 5 h.
Briefly, ET-1 and ET-2 were generated using a nine-step synthetic pathway with a 12% overall yield (Fig. 1). First, 4,9-dimethyl-9H-carbazole-3-carbaldehyde (1) was synthesized in five steps, according to the literature, starting from Hagemann’s ester (ethyl-2-methyl-4-oxocyclohex-2-enecarboxylate).37,38 We then generated N-methyl-5-demethyl ellipticine (ET-1) and 2-methyl-N-methyl-5-demethyl ellipticinium iodide (ET-2) in four subsequent steps.39 Aldehyde 1 was treated with aminoacetaldehyde diethylacetal in solvent-free conditions to yield imine 2. The imine was reduced with sodium borohydride to produce amine 3, which was treated with benzene sulfonyl chloride to produce sulfonamide 4. Finally, cyclization of ET-1 was achieved by treating sulfonamide 4 with hydrochloric acid in dioxane. ET-2 was obtained by treating ET-1 with iodomethane in dimethyl formamide. Stock solutions of the test compounds (50 mM) were prepared in DMSO. ET-1 and ET-2 both were more soluble than ellipticine.
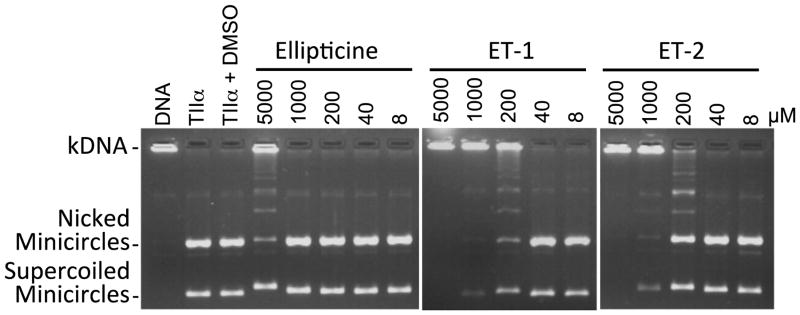
A number of assays were utilized to compare the effects of ET-1 and ET-2 on human topoisomerase IIα with those of the parent compound, ellipticine. First, the effects of the compounds on the overall catalytic activity of the enzyme were monitored using a decatenation assay (Fig. 2). Both ET-1 and ET-2 inhibited the ability of topoisomerase IIα to unlink kinetoplast DNA (kDNA) rings and blocked enzyme activity completely at concentrations of 200–1000 μM. In contrast, ellipticine required a concentration of >5000 μM to completely inhibit the decatenation activity of the type II enzyme.
Figure 2.
Effects of ellipticine, ET-1, and ET-2 (8–5000 μM) on DNA decatenation catalyzed by human topoisomerase IIα. Assays containing intact kDNA in the absence of topoisomerase IIα (DNA), or kDNA treated with topoisomerase IIα in the absence of ellipticine-based compounds (TIIα) or in the presence of compound diluent (TIIα + DMSO) are shown as controls. The positions of intact kDNA at the origin (kDNA), decatenated nicked kDNA minicircles, and decatenated supercoiled kDNA minicircles are indicated. Gels are representative of three independent experiments.
Although topoisomerase II poisons act by enhancing levels of DNA cleavage, they still have the capacity to inhibit overall enzyme activity.19,40 Therefore, to determine whether ET-1 and ET-2 act specifically as catalytic inhibitors or also have the capacity to act as topoisomerase II poisons, their effects on topoisomerase IIα-mediated DNA cleavage were assessed (Fig. 3). As reported previously, ellipticine was a modest inhibitor of DNA cleavage (IC50 >200 μM).1,28 Neither ET-1 nor ET-2 enhanced topoisomerase IIα-mediated DNA cleavage and both inhibited the DNA scission activity of the enzyme to a much greater extent than ellipticine. The IC50 for the two compounds were ~40 and 5 μM, respectively.
Figure 3.
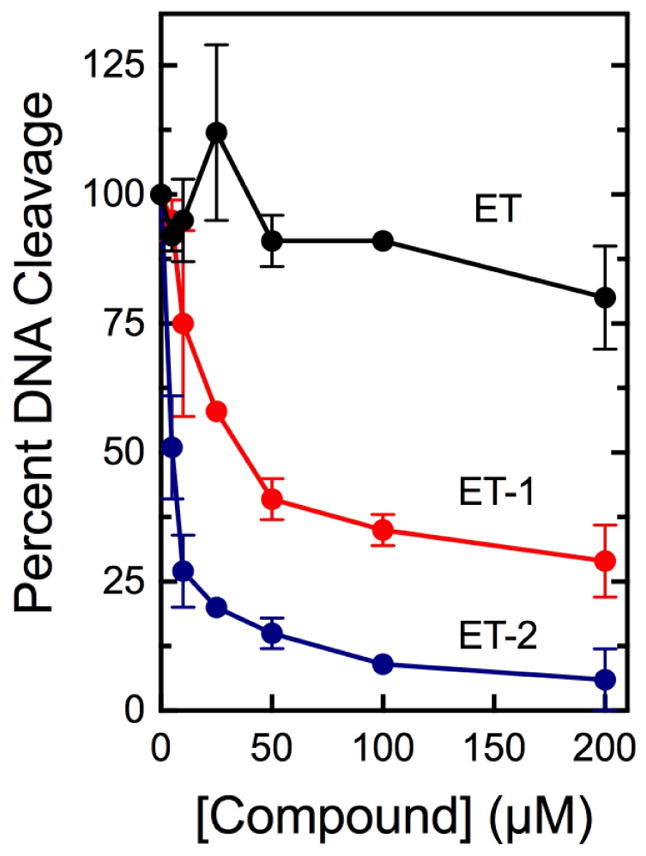
Effects of ellipticine, ET-1, and ET-2 on DNA cleavage mediated by human topoisomerase IIα. Results for ellipticine (ET; black), ET-1 (ET-1; red), and ET-2 (ET-2; blue) on the generation of enzyme-mediated double-stranded DNA breaks are shown. DNA cleavage levels were calculated relative to control reactions that contained no drug and were set to 100%. Error bars represent standard deviations for at least three independent experiments.
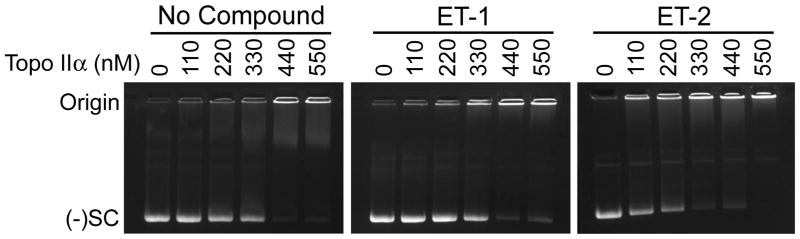
Catalytic inhibitors can block enzyme activity at a number of different steps of the topoisomerase II catalytic cycle.19–21 Compounds that inhibit the enzyme have been shown to act by blocking DNA binding, cleavage, or strand passage, or by interfering with ATP binding/hydrolysis. Because ET-1 and ET-2 inhibited DNA cleavage, we examined the effects of these compounds on topoisomerase IIα-DNA binding to determine whether the compounds are specific for DNA cleavage or act at a prior step in the catalytic cycle. ET-1 and ET-2 were used at 25 μM for this experiment, because DNA cleavage was inhibited substantially (~40 and 80% inhibition, respectively) at this concentration. Enzyme-DNA binding was monitored using an electrophoretic mobility shift assay (Fig. 4). Neither ET-1 nor ET-2 displayed any ability to inhibit topoisomerase IIα-DNA binding at the concentration employed. In fact, ET-2 appeared to enhance enzyme-DNA binding by ~50%. Thus, we conclude that ET-1 and ET-2 inhibit DNA cleavage without altering the ability of topoisomerase IIα to bind its DNA substrate.
Figure 4.
Effects of ET-1 and ET-2 on the binding of negatively supercoiled plasmid by human topoisomerase IIα. Results of an electrophoretic mobility shift assay are shown for an enzyme titration carried out in the presence of no compound, 25 μM ET-1, or 25 μM ET-2. Enzyme-DNA binding is indicated by the shift of the DNA from the position of negatively supercoiled [(−)SC] plasmid to the origin. Gels are representative of three independent experiments.
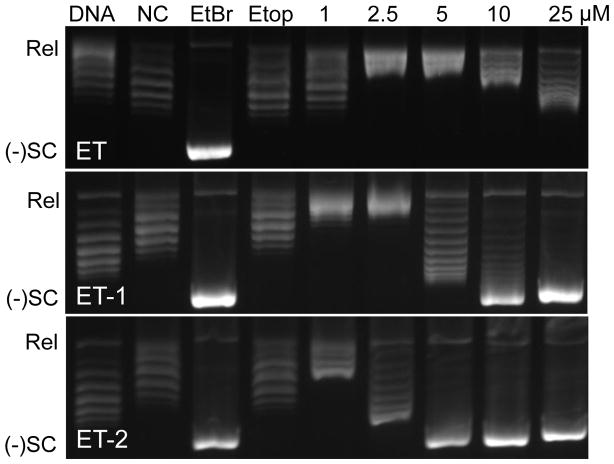
The ability of ellipticine to intercalate into DNA appears to play an important role in its antineoplastic activity.36 Because ET-1 and ET-2 are more potent inhibitors of human topoisomerase IIα than ellipticine, a topoisomerase I-DNA unwinding assay was utilized to compare the ability of these compounds to intercalate into relaxed plasmid DNA (Fig. 5). As determined by the shift in the plasmid from relaxed to negatively supercoiled DNA, all of the compounds intercalated into DNA. Similar to the inhibition of DNA cleavage observed with the compounds (see Fig. 3), the relative potency of intercalation was ET-2>ET-1≫ellipticine. This finding suggests that the ability of the ellipticine derivatives to inhibit the activity of topoisomerase IIα is related to their ability to intercalate into the double helix.
Figure 5.
Intercalation of ellipticine (ET), ET-1, and ET-2 into relaxed DNA. Results of a topoisomerase I-DNA unwinding assay are shown. Intercalation is indicated by the shift in the position of the plasmid from relaxed (Rel) to negatively supercoiled [(−)SC] DNA. A strong intercalator, ethidium bromide (10 μM), and a non-intercalator, etoposide (250 μM), are shown as positive and negative controls, respectively. Assays that contained only the relaxed DNA substrate (DNA) or relaxed DNA and topoisomerase I with no compound (NC) are shown. Gels are representative of three independent experiments.
There is a need for the development of new anticancer drugs. Given the demonstrated antineoplastic activity of ellipticine, derivatives of this compound have the potential to display greater activity against cells or enzyme targets.32,41,42 Results of the present study indicate that ET-1 and ET-2 are catalytic inhibitors of human topoisomerase IIα. Furthermore, both are more potent than ellipticine, and the activity of ET-1 and ET-2 toward the type II enzyme appears to be related to their enhanced ability to intercalate into DNA.
Supplementary Material
Acknowledgments
This research was supported by grant GM033944 (N.O.) from the National Institutes of Health and grant 115Z349 (Z.T.) from the Turkish Scientific and Technical Research Assembly. K.R.V. was a trainee under NIH grants R25-GM062459 and T32-GM08320 and was supported in part by a Research Supplement to Grant GM033944 to Promote Diversity in Health-Related Research. We are grateful to Rachel E. Ashley, Lorena Infante Lara, and Elizabeth G. Gibson for critical reading of the manuscript.
Footnotes
The authors declare no competing financial interests.
Experimental details for the synthesis and characterization of ET-1 and ET-2, as well as sources of materials and methods for biochemical assays are available in the accompanying Supplementary Data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Tewey KM, Chen GL, Nelson EM, Liu LF. J Biol Chem. 1984;259:9182. [PubMed] [Google Scholar]
- 2.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Biochem J. 1992;282(Pt 3):883. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Biochemistry. 2001;40:3316. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- 4.Bandele OJ, Clawson SJ, Osheroff N. Chem Res Toxicol. 2008;21:1253. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandele OJ, Osheroff N. Chem Res Toxicol. 2008;21:936. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ketron AC, Gordon ON, Schneider C, Osheroff N. Biochemistry. 2013;52:221. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senarisoy M, Canturk P, Zencir S, Baran Y, Topcu Z. Cell Biochem Biophys. 2013;66:199. doi: 10.1007/s12013-012-9468-5. [DOI] [PubMed] [Google Scholar]
- 8.Ashley RE, Osheroff N. Chem Res Toxicol. 2014;27:787. doi: 10.1021/tx400453v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ketron A, Osheroff N. Phytochem Rev. 2014;13:19. doi: 10.1007/s11101-013-9291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zupko I, Molnar J, Rethy B, Minorics R, Frank E, Wolfling J, Molnar J, Ocsovszki I, Topcu Z, Bito T, Puskas LG. Molecules. 2014;19:2061. doi: 10.3390/molecules19022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiborova M, Frei E. Curr Med Chem. 2014;21:575. doi: 10.2174/09298673113206660272. [DOI] [PubMed] [Google Scholar]
- 12.Vann KR, Sedgeman CA, Gopas J, Golan-Goldhirsh A, Osheroff N. Biochemistry. 2015;54:4531. doi: 10.1021/acs.biochem.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deweese JE, Osheroff MA, Osheroff N. Biochem Mol Biol Educ. 2008;37:2. doi: 10.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deweese JE, Osheroff N. Nucleic Acids Res. 2009;37:738. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitiss JL. Nat Rev Cancer. 2009;9:327. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Deibler RW, Chan HS, Zechiedrich L. Nucleic Acids Res. 2009;37:661. doi: 10.1093/nar/gkp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos SM, Tretter EM, Schmidt BH, Berger JM. Nat Rev Mol Cell Biol. 2011;12:827. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SH, Chan NL, Hsieh TS. Ann Rev Biochem. 2013;82:139. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- 19.Fortune JM, Osheroff N. Prog Nucleic Acid Res Mol Biol. 2000;64:221. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- 20.Larsen AK, Escargueil AE, Skladanowski A. Pharmacol Ther. 2003;99:167. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 21.Chene P, Rudloff J, Schoepfer J, Furet P, Meier P, Qian Z, Schlaeppi JM, Schmitz R, Radimerski T. BMC Chem Biol. 2009;9:1. doi: 10.1186/1472-6769-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pommier Y, Leo E, Zhang H, Marchand C. Chem Biol. 2010;17:421. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogorelcnik B, Perdih A, Solmajer T. Curr Pharm Des. 2013;19:2474. doi: 10.2174/1381612811319130016. [DOI] [PubMed] [Google Scholar]
- 24.Ketron AC, Osheroff N. Phytochem Rev. 2014;13:19. doi: 10.1007/s11101-013-9291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin S, Smith AF, Horning EC. J Am Chem Soc. 1959;81:1903. [Google Scholar]
- 26.Auclair C. Arch Biochem Biophys. 1987;259:1. doi: 10.1016/0003-9861(87)90463-2. [DOI] [PubMed] [Google Scholar]
- 27.Monnot M, Mauffret O, Simon V, Lescot E, Psaume B, Saucier JM, Charra M, Belehradek J, Jr, Fermandjian S. J Biol Chem. 1991;266:1820. [PubMed] [Google Scholar]
- 28.Fosse P, Rene B, Charra M, Paoletti C, Saucier JM. Mol Pharmacol. 1992;42:590. [PubMed] [Google Scholar]
- 29.Froelich-Ammon SJ, Patchan MW, Osheroff N, Thompson RB. J Biol Chem. 1995;270:14998. doi: 10.1074/jbc.270.25.14998. [DOI] [PubMed] [Google Scholar]
- 30.Cline SD, Macdonald TL, Osheroff N. Biochemistry. 1997;36:13095. doi: 10.1021/bi971770z. [DOI] [PubMed] [Google Scholar]
- 31.Garbett NC, Graves DE. Curr Med Chem Anticancer Agents. 2004;4:149. doi: 10.2174/1568011043482070. [DOI] [PubMed] [Google Scholar]
- 32.Kizek R, Adam V, Hrabeta J, Eckschlager T, Smutny S, Burda JV, Frei E, Stiborova M. Pharmacol Ther. 2012;133:26. doi: 10.1016/j.pharmthera.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Multon E, Riou JF, LeFevre D, Ahomadegbe JC, Riou G. Biochem Pharm. 1989;38:2077. doi: 10.1016/0006-2952(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 34.Rouesse J, Spielmann M, Turpin F, Le Chevalier T, Azab M, Mondesir JM. Eur J Cancer. 1993;29A:856. doi: 10.1016/s0959-8049(05)80424-1. [DOI] [PubMed] [Google Scholar]
- 35.Kattan J, Durand M, Droz JP, Mahjoubi M, Marino JP, Azab M. Am J Clin Oncol. 1994;17:242. doi: 10.1097/00000421-199406000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Paoletti C, Le Pecq JB, Dat-Xuong N, Juret P, Garnier H, Amiel JL, Rouesse J. Recent Res Cancer Res. 1980;74:107. doi: 10.1007/978-3-642-81488-4_15. [DOI] [PubMed] [Google Scholar]
- 37.Karmakar S, Dixit R, Nath A, Kumar S, Karmakar S. Indian J Pharmacol. 2012;44:131. doi: 10.4103/0253-7613.91887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ergün Y, Patir S, Okay G. J Heterocyclic Chem. 1998;35:1445. [Google Scholar]
- 39.Yokoyama Y, Okuyama N, Iwadate S, Momoi T, Murakami Y. J Chem Soc, Perkins Trans. 1;1990:1319. [Google Scholar]
- 40.McClendon AK, Osheroff N. Mutat Res. 2007;623:83. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stiborova M, Frei E. Curr Med Chem. 2014;21:575. doi: 10.2174/09298673113206660272. [DOI] [PubMed] [Google Scholar]
- 42.Russell EG, O’Sullivan EC, Miller CM, Stanicka J, McCarthy FO, Cotter TG. Invest New Drugs. 2014;32:1113. doi: 10.1007/s10637-014-0140-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.