Abstract
Western honey bees (Apis mellifera) face an increasing number of challenges that in recent years have led to significant economic effects on apiculture, with attendant consequences for agriculture. Nosemosis is a fungal infection of honey bees caused by either Nosema apis or N. ceranae. The putative greater virulence of N. ceranae has spurred interest in understanding how it differs from N. apis. Little is known of effects of N. apis or N. ceranae on honey bee learning and memory. Following a Pavlovian model that relies on the proboscis extension reflex, we compared acquisition learning and long-term memory recall of uninfected (control) honey bees versus those inoculated with N. apis, N. ceranae, or both. We also tested whether spore intensity was associated with variation in learning and memory. Neither learning nor memory differed among treatments. There was no evidence of a relationship between spore intensity and learning, and only limited evidence of a negative effect on memory; this occurred only in the co-inoculation treatment. Our results suggest that if Nosema spp. are contributing to unusually high colony losses in recent years, the mechanism by which they may affect honey bees is probably not related to effects on learning or memory, at least as assessed by the proboscis extension reflex.
Western honey bees (Apis mellifera) are ecologically and economically important pollinators world-wide, with pollination services contributing billions of dollars annually1,2. For reasons that appear multi-causal3,4,5,6, honey bee colonies have in recent years been suffering significant mortality in regions around the world, likely at an unprecedented rate7. Causes of mortality include pesticides, shortages of forage, improper management by beekeepers, and parasites8,9,10,11,12,13,14,15. Among the latter are two species of microsporidian fungi, Nosema apis and N. ceranae. Although many signs of pathology have been identified for infections with N. apis and N. ceranae, effects of these parasites on honey bee acquisition learning (hereafter, learning) and long-term memory recall (hereafter, memory) are not well studied. Wright (ref. 16) found that fungal infection by Metarhizium anisopliae was associated with both enhanced and impaired learning in honey bees, depending on a variety of other variables, including infection with Nosema apis. Here, we test directly whether Nosema spp. parasitism affects learning and memory in honey bees.
Nosema apis was the historic species infecting A. mellifera honey bees17, but probably early in this century, N. ceranae became an invasive parasite of A. mellifera, transferring from Asian honey bees A. cerana18,19,20,21,22,23. Currently, N. ceranae essentially matches N. apis’s nearly global distribution22, and the two species can co-infect honey bees9,22,24,25. Some theory predicts that co-infections select for increased virulence because of within-host competition for resources26,27,28. Although co-infections occur, N. ceranae has become the predominant species in many regions12,21,29,30, which suggests that N. ceranae may be a better competitor than N. apis24,31. This raises questions about the nature of differences between the two species; there is ongoing debate about which species is more virulent32,33,34, which could relate to competitive ability and explain higher mortality caused by N. ceranae than N. apis22,30.
Mortality from parasites may be a direct consequence of pathology to a host, or indirect wherein behaviour is modified. For example, parasitic infections can impair cognition, both in vertebrate35,36,37,38 and invertebrate hosts39,40,41,42. A Pavlovian classical conditioning model can be used to assess honey bee learning and memory43,44,45. The proboscis extension reaction (usually called a reflex; PER) is a sensory physiology paradigm in which honey bees learn to associate a neutral or conditioned stimulus (e.g., odour), with an unconditioned stimulus (e.g., sucrose). Learning is assumed when the conditioned stimulus elicits an extension of the proboscis43,44,45,46; under natural conditions the proboscis must be extended to enable a honey bee to drink. Memory is tested during extinction trials in which the conditioned stimulus is presented without the unconditioned stimulus.
Effects of N. apis and N. ceranae infections, acting singly or in co-infections, on honey bee learning and memory have not been assessed previously, although one study47 reported on reduced homing ability in N. ceranae-infected bees. We used PER to test if learning and memory were compromised in honey bees infected with Nosema, and if there were differences among N. apis, N. ceranae, and co-infections. Based on a hypothesis of increased virulence in co-infections, we predicted that honey bee learning and memory would be most significantly affected in bees infected with both N. apis and N. ceranae. Additionally, if N. ceranae is more virulent than N. apis, we predicted the former to have a more significant effect on learning and memory. We also tested whether greater infection intensity (spores per bee, hereafter spore intensity) had greater effects on learning and memory. Tests were performed 7 and 14 days post-inoculation (d.p.i.) to evaluate whether learning and memory were affected to a greater extent later on in infections as a consequence of cumulative pathology.
Results
General observations
In total, 577 honey bees were conditioned using PER; some mortality occurred before bees were ready for experiments, but sample sizes were roughly equal for each treatment. PCR-testing on 109 honey bees confirmed that no cross-contamination had occurred and that all co-infections were indeed co-infections. Few honey bees (N = 3, < 1%) responded spontaneously to geraniol (i.e., PER at first exposure to odour); these were removed from statistical analyses. Thirty-three percent (190 of 577) bees did not perform PER once (non-responders) during conditioning trials. Non-responders are usually assumed to have not learned associations, and are thus not tested in extinction trials48,49,50 (also see discussion in ref. 44).
Mean spore counts (in millions) by treatment 7 d.p.i were 0.1 in controls, 3.2 for N. apis, 2.0 for N. ceranae, and 2.7 in coinfections. Equivalent numbers 14 d.p.i. were 0.2, 23.7, 19.1, and 22.5. Spores observed in 29 control bees 7 d.p.i. and 23 control bees 14 d.p.i. were likely experimental artefacts that regularly arise in microscope work51.
Effects of treatments and spore intensities on learning and memory
At 7 d.p.i, spore intensities differed significantly among Nosema spp. treatments (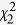 = 7.4, P = 0.03), with the N. ceranae treatment having significantly lower spore intensities than either N. apis- or co-inoculated bees (Fig. 1). Spore intensities increased from 7 d.p.i to 14 d.p.i. in all Nosema treatments (all Kruskal-Wallis
= 7.4, P = 0.03), with the N. ceranae treatment having significantly lower spore intensities than either N. apis- or co-inoculated bees (Fig. 1). Spore intensities increased from 7 d.p.i to 14 d.p.i. in all Nosema treatments (all Kruskal-Wallis 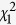 > 17.5, all Ps < 0.0001). At 14 d.p.i, there were no significant differences in spore intensities among treatments (Fig. 1).
> 17.5, all Ps < 0.0001). At 14 d.p.i, there were no significant differences in spore intensities among treatments (Fig. 1).
Figure 1. Log-transformed spore intensities in each Nosema treatment at 7 (left) and 14 (right) d post-inoculation.
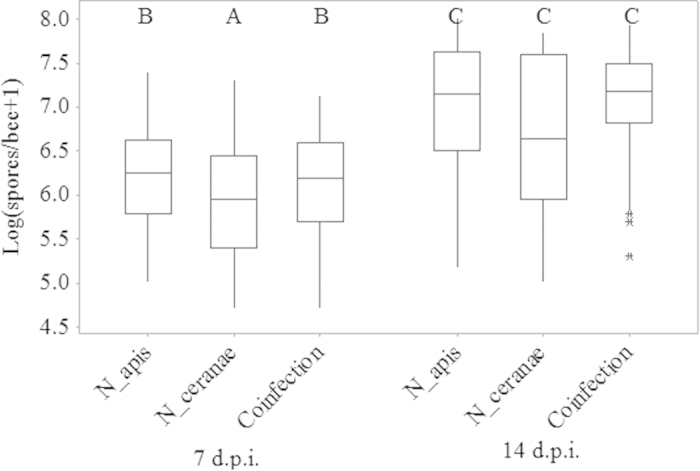
Sample sizes are given in Table 1. Treatments sharing letters were not statistically different (Mann-Whitney U tests). Boxplots show interquartile range (box), median (horizontal line within box), data range (vertical line above and below box), and outliers (asterisks).
There were no significant differences in learning or memory (both indexed by the number of positive PER responses; see methods) among treatments at either 7 or 14 d.p.i. (Table 1, Fig. 2). When all honey bees were pooled, learning 14 d.p.i. was significantly better than at 7 d.p.i. (Kruskal Wallis 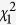 = 8.8, P = 0.003), but this pattern was not significant within treatment groups (all
= 8.8, P = 0.003), but this pattern was not significant within treatment groups (all 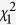 < 2.7, all P > 0.10). When all honey bees were pooled, there was no significant difference in memory between bees tested at 7 versus 14 d.p.i (
< 2.7, all P > 0.10). When all honey bees were pooled, there was no significant difference in memory between bees tested at 7 versus 14 d.p.i (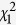 < 0.1, P = 0.95), nor were there differences within treatments (all
< 0.1, P = 0.95), nor were there differences within treatments (all 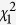 < 2.3, all P > 0.13).
< 2.3, all P > 0.13).
Table 1. Learning and memory (mean number of positive PERs in 8 trials for all bees within a treatment) did not differ among treatments 7 or 14 d post-inoculateion.
| Variable | Days post-inoculation | Treatment |
Kruskal-Wallis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Control |
N. apis |
N. ceranae |
Co-inoculation |
statistics |
|||||||
| N | 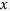 |
N | 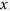 |
N | 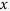 |
N | 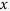 |
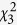 |
P | ||
| Learning | 7 | 89 | 2.7 | 85 | 2.2 | 80 | 2.2 | 83 | 2.6 | 2.9 | 0.40 |
| Learning | 14 | 68 | 3.4 | 64 | 2.7 | 38 | 2.6 | 46 | 3.2 | 4.7 | 0.20 |
| Memory | 7 | 82 | 0.6 | 62 | 0.5 | 65 | 0.6 | 69 | 0.8 | 2.9 | 0.41 |
| Memory | 14 | 58 | 0.6 | 48 | 0.5 | 28 | 0.3 | 37 | 0.4 | 5.1 | 0.17 |
N is total number of bees tested in each treatment (for 8 conditioning and 8 extinction trials).
Figure 2. Proportion of honey bees responding to odour presented with a sucrose reward for (conditioning trials 1–8) and to odour presented without a reward (extinction trials 9–16) relative to treatment.
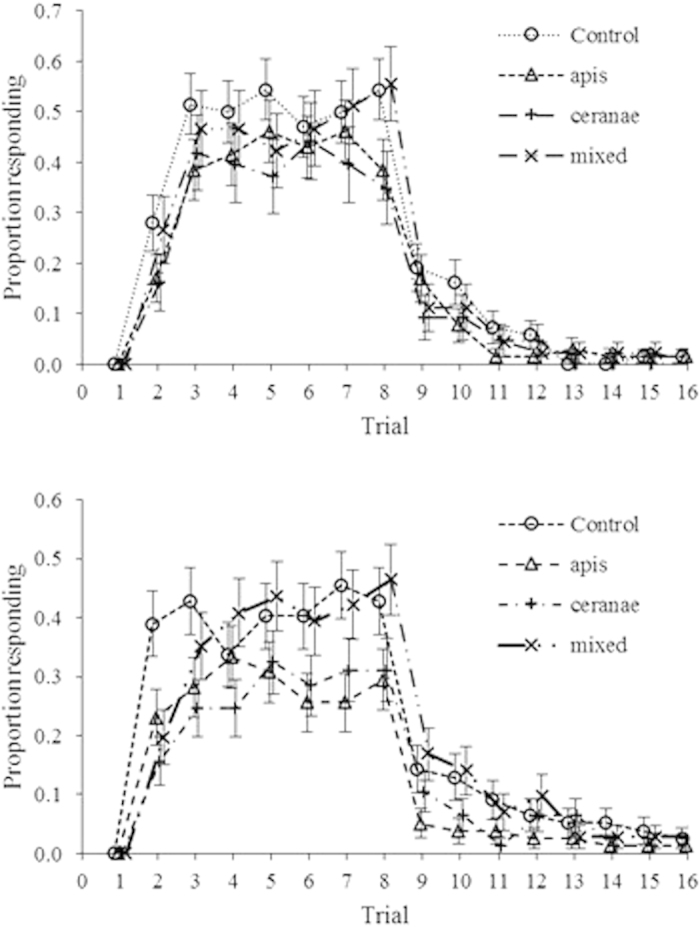
Sample sizes are given in Table 1.
Within Nosema treatments, two of 12 correlations between spore intensity and learning and memory were significant: N. apis-infected bees with higher spore intensities learned better 14 d.p.i. whereas co-inoculated honey bees had reduced memory 14 d.p.i. if they had higher spore intensities (Table 2).
Table 2. Within treatment Spearman correlations between spore intensities and learning and memory.
| Variable | Days post-inoculation |
Nosema apis |
Nosema ceranae |
Co-inoculation |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | rs | p | N | rs | p | N | rs | p | ||
| Learning | 7 | 85 | −0.08 | 0.47 | 80 | 0.10 | 0.07 | 83 | 0.09 | 0.43 |
| Memory | 7 | 82 | 0.02 | 0.86 | 65 | 0.39 | 0.59 | 69 | <0.01 | 0.99 |
| Learning | 14 | 64 | 0.21 | 0.02 | 38 | 0.14 | 0.39 | 46 | 0.09 | 0.57 |
| Memory | 14 | 48 | 0.10 | 0.90 | 28 | 0.11 | 0.56 | 37 | −0.32 | 0.05 |
Significant results in bold.
Discussion
Initially N. ceranae inoculations produced lower spore intensities compared to the other Nosema treatments, but spore intensities were equivalent among Nosema treatments by 14 d.p.i. Regardless, we found no differences in learning or memory among treatments either at 7 or 14 d.p.i., at least within the PER paradigm we used. We did observe better learning with higher spore intensities within N. apis-infected bees at 7 d.p.i., and poorer memory within co-inoculated bees with increased spore intensity at 14 d.p.i. The former result may indicate greater hunger and therefore more responsiveness52 whereas the latter result supports the hypothesis that co-infections result in increased virulence26. In 10 other tests, we observed no significant effects of spore intensity, so that on the whole we obtained limited evidence of effects of Nosema on learning and memory in honey bees, at least as assessed by PER.
Effects on learning and memory may vary with age, caste (e.g., nurse bee versus forager), satiation level, nutrition, experience, and genotype40,52,53. Moreover, differences in responsiveness between two genotypic strains can occur within 0 to 2 d of emergence. However, bees in this study were all of the Buckfast genetic strain, were sampled from the same colonies, emerged within a day or two of each other, received the same food, and experienced similar conditions in the cage prior to PER trials and in the trials themselves. Thus, we reduced potential influences on responses to rewards and increased our ability to detect possible effects on learning and memory.
Learning and memory were not significantly related to spore intensity among or within treatments in bees 7 d.p.i. One explanation for this is that Nosema spp. spores have not reached pathological levels by 7 d.p.i. This may explain why we observed no effects of spore intensity on honey bee learning and memory at 7 d.p.i., but did find some effects at 14 d.p.i.
Honey bees were tested at 7 and 14 d.p.i. to provide a range in spore intensity on which to test learning and memory and to evaluate effects of cumulative pathology. Temporal patterns we observed in spore intensities were consistent with other studies22,24. At 12 d.p.i., Forsgren and Fries (ref. 24) found N. ceranae and N. apis spores had roughly equal intensities, possibly due to lack of space for more spores in the ventriculus54. There is mixed evidence for whether one species of Nosema has a competitive advantage in co-infections30,31,54.
Reduced learning and memory could arise if parasites interfere with neural signalling processes40. Others55,56 have found that increased Nosema spp. spore intensity in bees was associated with energetic stress, which could affect neural signalling. In any case, increased consumption of food in response to parasitic infection is not uncommon in insects56 (but see ref. 57 for a review of vertebrates wherein anorexia is the dominant response to parasite infection). In previous studies, co-infected honey bees were significantly more responsive to sucrose and consumed significantly higher amounts, indicating increased appetite and overall hunger55,56.
Parasitism can disrupt ecologically significant components of cognition in animals36,37,38,58,59 and impairments to learning and memory could have significant detrimental effects on honey bee colony survival. However, our results provide only limited and contradictory evidence that Nosema spp. infections have damaging effects on learning and memory.
Methods
Source of spores
When spores are frozen, N. apis has higher rates of infectivity than N. ceranae60,61; therefore, frozen spores were used only to generate fresh spore stock for experimental inoculations (additional details in ref. 61). A spore homogenate was created from naturally infected dead, frozen honey bees collected in eastern Canada. Abdomens of 50 bees were added to 50 mL of distilled water and crushed using a mortar and pestle61. Homogenate suspensions were vortexed and viewed under phase-contrast light microscopy using a haemocytometer to count spores62,63 (Hausser Bright-Line, 1/400 cm, 0.1 mm depth). Homogenate was diluted with distilled water and assessed repeatedly using a haemocytometer until an equal spore amount of 125,000 spores per μl was achieved for each Nosema species. Duplex R-T PCR was performed following reference 25 to confirm species. Burgher-MacLellan et al.’s25 protocol allows one to distinguish N. apis, N. ceranae, and co-infections.
Source of honey bees for generation of fresh spore stock
Honey bees were collected from a colony in Coldbrook, Nova Scotia, Canada. Fifty honey bees were collected from hive entrances to first confirm that a colony was free of Nosema spp. To verify this, honey bees were freeze-killed, suspensions of their tissues created, and Nosema spp. spores counted, using a haemocytometer as above. In addition to being Nosema spp.-free, colonies had not been treated chemically against Nosema spp. or Shorten to V. destructor mites, limiting potential for chemotherapies to affect learning or memory64.
After confirming that honey bees were Nosema spp.-free, newly emerged honey bees from the same colony were used to generate fresh, even-aged spore stock for experimental inoculations65. A frame with brood that was 2–3 d before eclosion was placed in a mesh bag in a nucleus box and immediately transferred to a temperature-controlled and humidity-controlled growth chamber, maintained at 33o C and 45 ± 2% RH66.
Approximately 50 newly emerged honey bees were placed into each of two rectangular 17 × 12 × 13 cm plywood cages with removable Plexiglas sides and a wire mesh top. Honey bees were provided sucrose solution (50% w/w in water) administered ad libitum through a plastic syringe suspended from the wire mesh top of the cage. Food was removed after 2 d and honey bees were starved overnight in preparation for inoculations30.
Inoculation of honey bees to generate fresh spore stock
Honey bees were cooled in cages for ease of handling, grasped by the thorax with tweezers, and individually fed 5 μl of 50% w/w sucrose in water solution containing 125,000 spores per μl of either N. apis or N. ceranae61,67. After force-feeding, honey bees were fed ad libitum on sucrose solution and, in the following days, individual honey bees were selected, freeze-killed, and spore species confirmed as above. New spore homogenate was kept at room temperature for no more than an hour and used to inoculate experimental honey bees. Fresh spores were obtained for subsequent inoculations by crushing honey bees that had gone through PER trials (see below).
Preparing honey bees for PER testing
Frames with capped brood were collected according to previously described techniques on four occasions between July and September 2010 to provide newly emerged honey bees for inoculation and PER testing. At each occasion, after emergence, 20 honey bees were transferred to one of eight cages (same construction as above) with two cages [7- and 14-d post-inoculation (d.p.i.) honey bees] for each of four treatment groups: control (uninoculated), N. apis, N. ceranae, or co-inoculation. Honey bees were provided sucrose ad libitum for 2 d and then starved overnight in preparation for inoculations. Treatment groups were force fed 3 μl sucrose solution and 2 μl spore solution containing equal numbers of fresh spores as described above, achieved by dilutions41,53,68,69. The co-inoculated group received 1 μl each of N. apis and N. ceranae combined with 3 μl sucrose solution. Control honey bees were given sucrose solution to feed on ad libitum53. All cages were kept in a growth chamber as described above.
Conditioning and extinction trials
PER trials were run both 7 and 14 d.p.i. to assess learning and memory. Food was removed from cages the night before testing and the following morning, honey bees were cooled in their cages in a −20 oC freezer70, just until no visible signs of movement could be detected. Each honey bee was then loaded into a modified 1000-μl clear pipette tip with the tapered end removed and a small piece of wax securing the honey bee in place, so only the head was exposed and antennae and mouth parts were free to move70. Honey bees were randomly selected relative to parasite treatment, and PER was evaluated blind to treatment to avoid bias71.
Each honey bee’s PER responsiveness to sucrose was checked by applying 1.5 M sucrose solution, delivered on a wooden toothpick, to its left antenna72,73. A honey bee with a positive PER response (extension of proboscis) was fed sucrose for 3 sec and then left in darkness for 3 h. Honey bees that failed to respond were not used further in PER testing72,73,74.
Each bee received continuous air flow for 15 sec to acclimate it to mechanosensory stimulation. A manual valve controlled continuous air flow and delivery of the stimulus odour; both united at a mixing chamber positioned 10 to 15 mm in front of a honey bee’s head. A vacuum system behind the honey bee continuously removed odour from the testing area and contributed to drawing air and odour over honey bee antennae. Air was dispensed at a rate of approximately 1.0 L per min. Honey bees that spontaneously extended their proboscis in the first trial of the learning phase (air flow or before presentation of sucrose) were taken out of the experiment because this response indicates a previously established odour/reward association70,75. In each conditioning trial, honey bees were presented with the odour geraniol followed by a sucrose reward. Geraniol is common in many plant oils and is produced by the Nasonov gland and used as an attraction signal in worker bees72,76. Additionally, floral odours are learned faster than other odours77 and thus, are often used in conditioning experiments. The conditioned stimulus was prepared by pipetting 3 μl of geraniol onto filter paper that was housed in a syringe44,78,79,80.
Immediately following the 15-sec acclimation period, the conditioned stimulus was delivered for 6 sec. Three seconds after the onset of odour, sucrose (unconditioned stimulus) was delivered to the left antenna using a wooden toothpick for 1 sec and then to the proboscis for 2 sec of feeding70,72. A positive PER was recorded when the mandibles opened and the proboscis extended in response to the odour but before sucrose delivery; this indicated a learned response. The interval between two successive trials was 9 min during which time we tested the other bees that had been prepared. There were 8 conditioning trials/bee so that a score of 8 indicated maximum learning. Following 8 trials, honey bees were fed to satiation and kept in darkness at room temperature for 24 h. After these 24 h, 8 extinction trials were done to test memory; these were the same as conditioning trials but without a sucrose reward.
Individuals were scored for the number of times they exhibited PER in response to the odour in conditioning and extinction trials. Following extinction trials, honey bees were freeze-killed and spore counts carried out as above. Conventional PCR was completed on all honey bees in the co-inoculated treatment and a random sample of N. apis and N. ceranae treatments to confirm that no cross-contamination had occurred.
Statistical analyses
Statistical analyses were conducted in SAS version 9.3 (Cary, North Carolina). Data were not normally distributed (Kolmogorov-Smirnov tests for normality) even after transformations; thus, non-parametric tests of raw data were done. Spore intensities, learning, and memory were compared among treatments using Kruskal-Wallis tests, following up with Mann-Whitney U tests where significance was obtained. We tested whether learning and memory were related to spore intensity using Spearman’s rank correlations. Spore intensities, learning, and memory were also compared between 7 and 14 d.p.i. using Kruskal-Wallis tests.
Additional Information
How to cite this article: Charbonneau, L. R. et al. Effects of Nosema apis, N. ceranae, and coinfections on honey bee (Apis mellifera) learning and memory. Sci. Rep. 6, 22626; doi: 10.1038/srep22626 (2016).
Acknowledgments
We thank Kevin Spicer for providing colonies from which bees were obtained, and Team Shutler for critical evaluations at all stages. Research was supported by a Natural Sciences and Engineering Research Council of Canada Industrial Postgraduate Scholarship to L.R.C. and to G.R.W. and Acadia University Research Funds to D.S. We appreciate the input of a number of reviewers.
Footnotes
Author Contributions L.R.C., D.S. and G.R.W. developed ideas and also methods with N.K.H., all authors were involved in applying for funding, L.R.C. conducted the bulk of the data collection, L.R.C. and D.S. analyzed the data, and L.R.C., N.K.H., G.R.W. and D.S. were involved in writing and editing.
References
- Morse R. A. & Calderone N. W. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture 128, 1–15 (2000). [Google Scholar]
- Gallai N., Salles J. M., Settele J. & Vaissiere B. E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821 (2009). [Google Scholar]
- Watanabe M. Colony collapse disorder: Many suspects, no smoking gun. Bioscience 58, 384–388 (2008). [Google Scholar]
- Le Conte Y., Ellis M. & Ritter W. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41, 353–363 (2010). [Google Scholar]
- vanEngelsdorp D. & Meixner , M. D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103, S80–S90 (2010). [DOI] [PubMed] [Google Scholar]
- Williams G. R. et al. Colony Collapse Disorder in context. Bioessays 32, 845–846 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P. & Carreck N. L. Honey bee colony losses. J. Apicult. Res. 49, 1–6 (2010). [Google Scholar]
- Desneux N., Decourtye A. & Delpuech J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honeybees. J. Invertebr. Pathol. 101, 204–209 (2009). [DOI] [PubMed] [Google Scholar]
- Alaux C., Ducloz F., Crauser D. & Le Conte Y. Diet effects on honeybee immunocompetence. Biol. Lett. 6, 562–565 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacandritsos N. et al. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 105, 335–340 (2010). [DOI] [PubMed] [Google Scholar]
- Chen Y. P. & Huang Z. Y. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie 41, 364–374 (2010). [Google Scholar]
- Currie R. W., Pernal S. F. & Guzmán-Novoa E. Honey bee colony losses in Canada. J. Apicult. Res. 49, 104–106 (2010). [Google Scholar]
- Potts S. G. et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- Runckel C. et al. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 6 e20656 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. The effects of pathogens on honeybee learning and foraging behaviour. PhD thesis, Univ. Warwick (2013).
- Matheson A. World bee health update. Bee World 76, 31–39 (1996). [Google Scholar]
- Fries I., Feng F., daSilva A., Slemenda S. B. & Pieniazek N. J. Nosema ceranae n. sp. Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis ceranae (Hymenoptera, Apidae). Eur. J. Protistol. 32, 356–365 (1996). [Google Scholar]
- Higes M., Martín R. & Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92, 93–95 (2006). [DOI] [PubMed] [Google Scholar]
- Huang W. F., Jiang J. H., Chen Y. W. & Wang C. H. A. Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 38, 30–37 (2007). [Google Scholar]
- Klee J. et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 96, 1–10 (2007). [DOI] [PubMed] [Google Scholar]
- Paxton R. J., Klee J., Korpela S. & Fries I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38, 558–565 (2007). [Google Scholar]
- Williams G. R., Shafer A. B. A., Rogers R. E. L., Shutler D. & Stewart D. T. First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in Canada and central U.S.A. J. Invertebr. Pathol. 97, 189–192 (2008). [DOI] [PubMed] [Google Scholar]
- Forsgren E. & Fries I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 170, 212–217 (2010). [DOI] [PubMed] [Google Scholar]
- Burgher-MacLellan K. L., Williams G. R., Shutler D., MacKenzie K. & Rogers R. E. L. Optimization of duplex real-time PCR with melting-curve analysis for detecting the microsporidian parasites Nosema apis and Nosema ceranae in Apis mellifera. Can. Ent. 142, 271–283 (2010). [Google Scholar]
- Poulin R. Evolutionary Ecology of Parasites, 2nd ed. Princeton University Press, New Jersey (2007). [Google Scholar]
- Choisy M. & deRoode J. C. Mixed infections and the evolution of virulence: Effects of resource competition, parasite plasticity, and impaired host immunity. Am. Nat. 175, E105–E118 (2010). [DOI] [PubMed] [Google Scholar]
- Bashey F., Reynolds C., Sarin T. & Young S. K. Virulence and competitive ability in an obligately killing parasite. Oikos 120, 1539–1545 (2011). [Google Scholar]
- Williams G. R., Sampson M. A., Shutler D. & Rogers R. E. L. Does fumagillin control the recently-detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 99, 342–344 (2008). [DOI] [PubMed] [Google Scholar]
- Williams G. R., Shutler D., Burgher-MacLellan K. L. & Rogers R. E. L. Infra-population and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS One 9, e99465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsopoulou M. E., McMahon D. P., Doublet V., Bryden J. & Paxton R. J. Interspecific competition in honeybee intracellular gut parasites is asymmetric and favours the spread of an emerging infectious disease. Proc. Roy. Soc. B: Biol. Sci. 282, 20141896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster D. L. et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318, 283–287 (2007). [DOI] [PubMed] [Google Scholar]
- Guzmán-Novoa E. et al. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41, 443–450 (2010). [Google Scholar]
- Higes M., Meana A., Bartolomé C. & Botías C. & Martín‐Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Env. Microbiol. Reports 5, 17–29 (2013). [DOI] [PubMed] [Google Scholar]
- Braithwaite V. A. et al. Spatial and discrimination learning in rodents infected with the nematode Strongyloides ratti. Parasitology 117, 145–154 (1998). [DOI] [PubMed] [Google Scholar]
- Cox D. M. & Holland C. V. Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory in the murine host. J. Helminthol. 75, 33–41 (2001). [DOI] [PubMed] [Google Scholar]
- Kavaliers M. & Colwell D. D. Reduced spatial learning in mice infected with nematode, Heligmosomoides polygyrus. Parasitology 110, 591–597 (1995). [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Colwell D. D. & Galea L. A. M. Parasitic infection impairs spatial learning in mice. Anim. Behav. 50, 223–229 (1995). [Google Scholar]
- Gegear R. J., Otterstatter M. C. & Thomson J. D. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. Roy. Soc. B. 273, 1073–1078 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J. & Mueller U. Virus infection causes specific learning deficits in honeybee foragers. Proc. Roy. Soc. B. 274, 1517–1521 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj J. & Fuchs S. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37, 577–587 (2006). [Google Scholar]
- Kralj J., Brockmann A., Fuchs S. & Tautz J. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Compar. Physiol. A. 193, 363–370 (2007). [DOI] [PubMed] [Google Scholar]
- Hammer M. & Menzel R. Learning and memory in the honeybee. J. Neurosci. 15, 1617–1630 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E. H., Shutler D. & Hillier N. K. The proboscis extension reflex to evaluate learning and memory in honeybees (Apis mellifera): Some caveats. Naturwissenschaften 99, 677–686 (2012). [DOI] [PubMed] [Google Scholar]
- Giurfa M. & Sandoz J. C. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learning Memory 19, 54–66 (2012). [DOI] [PubMed] [Google Scholar]
- Alloway T. M. Learning and memory in insects. Annu. Rev. Entomol. 17, 43–56 (1972). [Google Scholar]
- Wolf S. et al. So near and yet so far: harmonic radar reveals reduced homing ability of Nosema infected honeybees. PloS One 9, e103989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R., Page R. E. & Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol. Learning Memory 76, 138–150 (2001). [DOI] [PubMed] [Google Scholar]
- Latshaw J. S. & Smith B. H. Heritable variation in learning performance affects foraging preferences in the honey bee (Apis mellifera). Behav. Ecol. Sociobiol. 58, 200–207 (2005). [Google Scholar]
- Déglise P., Dacher M., Dion E., Gautier M. & Armengaud C. Regional brain variations of cytochrome oxidase staining during olfactory learning in honeybees (Apis mellifera). Behav. Neurosci. 117, 540–547 (2003). [DOI] [PubMed] [Google Scholar]
- Weber M. Philosophy of Experimental Biology. Cambridge University Press, New York (2005). [Google Scholar]
- Mayack C. & Naug D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 100, 185–188 (2009). [DOI] [PubMed] [Google Scholar]
- Naug D. & Gibbs A. Behavioral changes mediated by hunger in honeybees infected with N. ceranae. Apidologie 40, 595–599 (2009). [Google Scholar]
- Pankiw T. & Page R. E. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A. 185, 207–213 (1999). [DOI] [PubMed] [Google Scholar]
- Pankiw T., Tarpy D. R. & Page R. E. Genotype and rearing environment affect honeybee perception and foraging behaviour. Animal Behaviour, 64, 663–672. [Google Scholar]
- Fries I. 1988. Infectivity and multiplication of Nosema apis Z. in the ventriculus of the honey bee. Apidologie 19, 319–328 (2002). [Google Scholar]
- Kyriazakis I., Tolkamp B. J. & Hutchings M. R. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 56, 265–274 (1998). [DOI] [PubMed] [Google Scholar]
- Gibertini M., Newton C., Friedman H. & Klein T. W. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-β. Brain Behav. Immun. 9, 113–128 (1995). [DOI] [PubMed] [Google Scholar]
- Libersat F., Delago A. & Gal R. Manipulation of host behavior by parasitic insects and insect parasites. Annu. Rev. Entomol. 54, 189–207 (2009). [DOI] [PubMed] [Google Scholar]
- Fries I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 103, S73–S79 (2010). [DOI] [PubMed] [Google Scholar]
- Fries I. et al. Standard methods for Nosema research. J. Apicult. Res. 51(5) (2013). [Google Scholar]
- Cantwell G. E. Standard methods for counting Nosema spores. Am. Bee J. 110, 222–223 (1970). [Google Scholar]
- Rogers R. E. L. & Williams G. R. Monitoring Nosema disease in honey bee colonies. Bee Culture 135, 19–21 (2007). [Google Scholar]
- Frost E. H., Hillier N. K. & Shutler D. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exper. Biol. 216, 2931–2938 (2013). [DOI] [PubMed] [Google Scholar]
- Higes M., Garcia-Palencia P., Martín-Hernández R. & Meana A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94, 211–217 (2007). [DOI] [PubMed] [Google Scholar]
- Kraus B. & Velthuis H. H. W. High humidity in the honey bee (Apis mellifera L) brood nest limits reproduction of the parasitic mite Varroa jacobsoni Oud. Naturwissenschaften 84, 217–218 (1997). [Google Scholar]
- Hassanein M. H. Studies on the effect of infection with Nosema apis on the physiology of the queen honey-bee. Quart. J. Microscop. Sci. 92, 225–231 (1951). [Google Scholar]
- Campbell J., Kessler B., Mayack C. & Naug D. Behavioural fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology 137, 1487–1491 (2010). [DOI] [PubMed] [Google Scholar]
- Huang W. F. & Solter L. F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 113, 35–41 (2013). [DOI] [PubMed] [Google Scholar]
- Bitterman M. E., Menzel R., Fietz A. & Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Compar. Psychol. 97, 107–119 (1983). [PubMed] [Google Scholar]
- Rosenthal R. & Fode K. L. The effect of experimenter bias on the performance of the albino rat. Behav. Sci. 8, 183–89 (1963). [Google Scholar]
- Getz W. M. & Smith K. B. Olfactory sensitivity and discrimination of mixtures in the honeybee Apis mellifera. J. Comp. Physiol. A. 160, 239–245 (1987). [Google Scholar]
- Gil M. & De Marco R. J. Olfactory learning by means of trophallaxis in Apis mellifera. J. Exper. Biol. 208, 671–680 (2005). [DOI] [PubMed] [Google Scholar]
- Giurfa M. Behavioural and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A. 193, 801–824 (2007). [DOI] [PubMed] [Google Scholar]
- Mercer A. R. & Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J. Comp. Physiol. A. 145, 363–368 (1982). [Google Scholar]
- Trhlin M. & Rajchard J. Chemical communication in the honeybee (Apis mellifera L.): a review. Veterinarni Medicina 56, 265–273 (2011). [Google Scholar]
- Menzel R. Neurobiology of learning and memory: the honeybee as a model system. Naturwissenschaften 70, 504–511 (1983). [DOI] [PubMed] [Google Scholar]
- Frost E. H., Shutler D. & Hillier N. K. Effects of cold immobilization and recovery period on honeybee learning, memory, and responsiveness to sucrose. J. Insect Physiol. 57, 1385–1390 (2011). [DOI] [PubMed] [Google Scholar]
- Qin Q. H., He X. J., Tian L. Q., Zhang S. W. & Zeng Z. J. Comparison of learning and memory of Apis cerana and Apis mellifera. J. Comp. Physiol. A. 198, 777–786 (2012). [DOI] [PubMed] [Google Scholar]
- Getz W. M., Brückner D. & Smith K. B. Conditioning honeybees to discriminate between heritable odors from full and half-sisters. J. Comp. Physiol. A. 159, 251–256 (1986). [Google Scholar]


