Abstract
Mycobacterium ulcerans disease (Buruli ulcer) is a serious ulcerating skin disease which is common in many tropical countries. Standard treatment, by extensive excision and skin grafting, is not available in rural communities where the disease is common. We evaluated the efficacy and safety of treatment with topical nitrogen oxides. Thirty-seven patients with a clinical diagnosis of Buruli ulcer caused by M. ulcerans disease were randomly assigned to one of two groups. In one group, two creams containing sodium nitrite (6%, wt/wt) or citric acid monohydrate (9%, wt/wt) were applied daily for 6 weeks, while the other group received a placebo. In the second 6 weeks, both groups received the nitrogen oxide-generating combination of creams. Treatment was continued for another 4 weeks for patients whose ulcers were not healed after 12 weeks. The ulcer surface area was monitored by weekly tracings made by assessors blinded to the treatment. In the first 6 weeks, patients on sodium nitrite and citric acid monohydrate (group I, active treatment) showed a rapid decrease in ulcer size from 28.6 ± 5.6 cm2 (mean ± standard error) to 12.6 ± 3.2 cm2, a decrease significantly greater than that in group II (from 15.3 ± 3.1 to 11.7 ± 3.7 cm2; P = 0.03). Five ulcers in the placebo group enlarged during this period, compared with one in the active group. In the second 6 weeks (both groups on active treatment), the rates of healing were similar for the two groups and there was a significant reduction in ulcer size in group II (previously on placebo) compared to the first 6 weeks. Yellow pigmentation of the skin, which disappeared 3 days after treatment was stopped, was the only side effect to date. We conclude that creams releasing nitrogen oxides increase the healing rate of ulcers caused by M. ulcerans infection with minimal adverse events. This is the first controlled trial of any form of therapy which demonstrates efficacy in treating this disease.
Mycobacterium ulcerans infects the skin, and its toxin causes extensive subcutaneous necrosis, leading to the formation of ulcers with undermined edges known as Buruli ulcer. It is a common and serious disease in some parts of the tropics, particularly in West Africa (21). Surgical excision of early lesions of M. ulcerans disease is the standard treatment and is often curative, but many patients do not present until there is extensive ulceration requiring debridement with wide excision and skin grafting. This expensive treatment is not available in most areas where the disease is endemic (3). Chemotherapy with antimicrobial agents has been used (11), but the only published controlled trials suggest that clofazamine is ineffective (19) and that rifampin and dapsone combined have limited efficacy (9). Many types of topical treatment have been reported, but none has been subjected to the rigors of a controlled clinical trial (2).
Acidification of nitrite produces nitrous acid, which rapidly decomposes to form oxides of nitrogen, one of which is nitric oxide, as shown in equations 1 to 3.
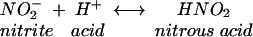 |
(1) |
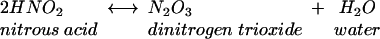 |
(2) |
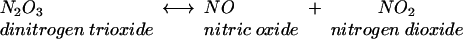 |
(3) |
The overall reaction is expressed by equation 4.
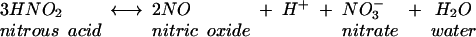 |
(4) |
In vitro, nitric oxide plays an important role in the killing of Escherichia coli (13, 16), Candida spp. (6, 12), Leishmania spp. (15, 23), and Mycobacterium leprae (1). Staphylococcus aureus and Propionibacterium acnes are also inhibited by nitric oxide (14, 24). Tinea pedis, caused by the fungi Trichophyton rubrum and Trichophyton mentagrophytes, has been treated effectively by topical nitrogen oxides generated by acidified nitrite (26).
We carried out an open-label study on 12 patients with Buruli ulcer from the Atwima District of the Ashanti Region in Ghana. That study suggested that topical treatment of M. ulcerans disease with creams which generate nitrogen oxides when mixed in situ can heal most established Buruli ulcers with diameters of less than 15 cm after administration on 6 days per week for 7 to 9 weeks (unpublished data; patients not included in the present study). Topical treatment with nitric oxide-generating creams has considerable advantages over surgery or antibiotic therapy because of the practical simplicity of their application. In view of the encouraging results from our earlier study, we designed a randomized, double-blind pilot trial of nitrogen oxide-releasing acidified nitrite creams compared to placebo creams in order to establish whether a healing effect could be demonstrated and to assess the safety of this form of treatment for Buruli ulcer.
MATERIALS AND METHODS
The study protocol was approved by the ethics committees (institutional review boards) of the School of Medical Sciences, Kumasi, Ghana, and the St. George's Hospital National Health Service Trust, London, United Kingdom. In November 2001, patients with a clinical diagnosis of M. ulcerans disease were recruited from the Atwima District of the Ashanti Region in Ghana by village health workers experienced in diagnosing Buruli ulcer. Patients were screened by the principal investigator (R.P.) and were included if they were aged 5 years or more and had ulcers with a maximum diameter of 15 cm that were clinically compatible with M. ulcerans disease, that is, painless, full-thickness skin ulcers with undermined edges and with no evidence of bacterial superinfection or systemic disease. Exclusion criteria included edematous forms of the disease, lesions involving the genitals and eyes, excessive alcohol intake, and treatment with antibiotics or immunomodulatory drugs within the previous month. Participants were withdrawn from the study if there were serious adverse events, if they were not compliant with the protocol, or if there was rapid enlargement of the lesion to twice its original diameter, in which case they were referred for surgery.
Consent and randomization.
Informed consent was obtained after the study had been explained to participants and their relatives by the principal investigator, speaking in their own language (Twi). In the case of children, consent was obtained from their parents or guardians. Patients were numbered consecutively from 1 to 38 and randomized to receive active or placebo treatment for the first 6 weeks according to a computer-generated sequence. One patient was randomized in error and was withdrawn prior to treatment. Investigators and participants remained blinded to the nature of the treatment throughout the study.
Diagnosis.
Punch biopsy specimens (diameter, 4 mm) from the edge of the ulcer were obtained from each study participant for Ziehl-Neelsen staining, culture, and histological examination. For cultivation, homogenized biopsy specimens (1 ml), decontaminated by the modified Petroff method (4) for 10 min, were inoculated on Löwenstein-Jensen medium. Cultures were incubated at 31°C and examined weekly for 6 months before they were discarded.
Intervention.
Active creams contained sodium nitrite (6%, wt/wt) in one tube and citric acid monohydrate (9%, wt/wt) in the other, both in a base of aqueous cream. Placebo creams contained only the aqueous cream base. Creams were supplied in 30-g tubes, each labeled with a patient identification number. For the first 6 weeks, participants in group I received active creams while those in group II received placebo creams in a double-blind manner. In the subsequent 6 weeks, both groups received treatment with active creams. All participants whose ulcers had improved after 12 weeks were offered active treatment for a further 4 weeks. Participants attended a dedicated outpatient clinic at Komfo Anokye Teaching Hospital on 6 days per week, and a maximum of 1 g of each cream was applied and mixed together in situ by one of two trained research nurses. A dry gauze dressing was applied, with instructions for it to be left undisturbed until the next application. Patients were assessed daily for adverse events.
At baseline and at weekly intervals, the investigators (R.P. and O.A.) assessed the appearance of ulcers and measured the ulcerated areas by tracing onto acetate sheets. Photographs were taken weekly with a Macro 3 Polaroid camera.
Outcome measures.
The efficacy end points were complete healing or significant reduction in the surface area of lesions. Adverse events were recorded daily.
Statistical analysis.
Tracings of ulcers were scanned into a computer, and the surface area was estimated by using Scion Corporation imaging software. Two readings were taken for each ulcer by two independent observers, and the results were averaged. P values were calculated where necessary with a Gaussian approximation. The Mann-Whitney and sign nonparametric tests were used to compare the changes in size of the ulcers in the two groups. All statistical tests were two sided. Analysis was performed using SAS software (20).
RESULTS
Patients.
Thirty-seven patients were randomized, but seven patients were not analyzed for the following reasons (one patient each): excessive alcohol intake, surgery on the ulcer after the first week to release contracture (not reported to investigators), development of edematous M. ulcerans disease in the first week, ulcer outside protocol definition (too large), failure to adhere to the protocol, age less than 5 years, and bone involvement which required surgery 1 week after enrollment. The characteristics of the remaining 30 patients, 16 in group I and 14 in group II, are shown in Table 1. The groups were similar in terms of sex and treatment prior to enrollment (daily dressings with Eusol, a solution of chlorinated lime and boric acid), but there were differences in age, duration of lesion, and size and site of lesion. The median age of group I (active treatment) was 40 years (range, 9 to 90 years), while that of group II was 14 years (range, 5 to 65 years). Fifty percent of the ulcers in group I and 78.6% of the ulcers in group II had been present for less than 1 year. Ulcers in group I were bigger at baseline than those in group II (median surface area, 22.3 cm2 [range, 0.9 to 69.3 cm2] versus 15.2 cm2 [range, 1.0 to 37.8 cm2], respectively). There were more lesions on the lower limbs (87.5%) in group I than in group II (64.3%).
TABLE 1.
Baseline characteristics of the 30 patients who completed the protocol
| Characteristic | Value for:
|
|
|---|---|---|
| Group I (active) | Group II (placebo) | |
| Total no. (%) | 16 (100) | 14 (100) |
| Age | ||
| 5-15 yr | 4 (25) | 10 (71.4) |
| >15-50 yr | 6 (37.5) | 1 (7.1) |
| >50 yr | 6 (37.5) | 3 (21.4) |
| Median (yr) | 40 | 14 |
| Range (yr) | 9-90 | 5-65 |
| Sex | ||
| Male | 8 (50.0) | 5 (35.7) |
| Female | 8 (50.0) | 9 (64.3) |
| Duration of ulcer | ||
| <1 yr | 8 (50) | 11 (78.6) |
| ≥1 yr | 8 (50) | 3 (21.4) |
| Site of lesion | ||
| Upper limb | 2 (12.5) | 3 (21.4) |
| Lower limb | 14 (87.5) | 9 (64.3) |
| Trunk | 0 (0.0) | 2 (14.3) |
| Baseline surface area of ulcer (cm2) | ||
| Mean ± SE | 28.6 ± 5.6 | 15.3 ± 3.1 |
| Median | 22.3 | 15.2 |
| Range | 0.9-69.3 | 1.0-37.8 |
| Treatment prior to enrollment in study | ||
| Eusol dressings | 10 (62.5) | 12 (85.7) |
| Topical antibiotic (metronidazole or penicillin) | 2 (12.5) | 1 (7.1) |
| Topical herbs | 0 | 1 (7.1) |
| Not known | 4 (25) | 0 |
Efficacy.
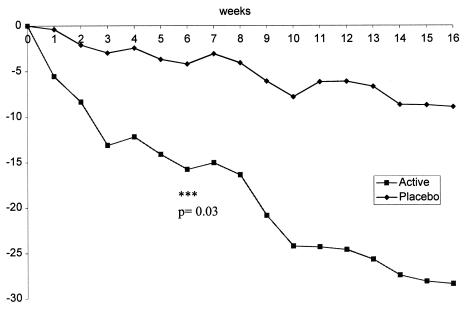
Analysis of the population by protocol showed a rapid decrease in the sizes of ulcers in group I during the first 6 weeks which was significantly greater than that in group II (Fig. 1). The baseline ulcerated surface area was reduced from 28.6 ± 5.6 cm2 (mean ± standard error) to 12.6 ± 3.2 cm2 in group I and from 15.3 ± 3.1 to 11.7 ± 3.7 cm2 in group II (P = 0.03 for the difference between groups by the Mann-Whitney U test). This change was most marked in the first 3 weeks. During weeks 6 to 12, when group II received active treatment, there was a significant reduction in the size of the ulcerated area, from 11.7 ± 3.7 to 7.2 ± 2.7 cm2 (P = 0.01 by the sign test), whereas the change for this group during the first 6 weeks was not significant (from 15.3 ± 3.1 to 11.7 ± 3.7 cm2; P = 0.19).
FIG. 1.
Changes with time in mean surface areas (in square centimeters) of Buruli ulcers treated with acidified nitrite creams (active treatment, group I) or placebo creams (group II). Asterisks indicate significant difference between groups at the end of the first 6 weeks.
Five (35.7%) ulcers in group II increased in size during the first 6 weeks, as opposed to only one (6.3%) in group I. One ulcer in group I and two in group II were larger after 16 weeks than at baseline. The mean reduction in size of the other ulcers after 6 weeks was 68% in group I and 35% in group II. After 16 weeks, the mean reductions for groups I and II were 83 and 90%, respectively.
During weeks 6 to 12, the rates of healing were similar in the two groups, and after 16 weeks, 4 out of 16 ulcers in group I and 6 out of 14 in group II had healed completely. Twenty-six (86.7%) of the 30 ulcers had healed by more than 50% after 16 weeks, and the mean levels of healing of these ulcers were 85% for group I and 90% for group II.
The diagnosis of M. ulcerans disease was confirmed by staining for acid-fast bacilli and culture for four patients, all of whom improved on active treatment, with reduction of the ulcerated area by 95 and 93% over 10 weeks for two patients and by 72 and 100% over 16 weeks for the other two patients. The remainder, however, were negative by both methods. All punch biopsy specimens showed nonspecific inflammation on histology.
Adverse reactions.
Acidified nitrite creams were well tolerated. All patients on both active and placebo creams developed a yellow pigmentation at the ulcer site after application of the cream, which disappeared when treatment was discontinued. There were no other side effects.
One patient, aged 90 years, died during week 13 from lobar pneumonia, which was thought to be unrelated to the study. Another patient developed pain in the ulcer at 12 weeks, and cellulitis was noted. E. coli and S. aureus were isolated from a wound swab, and appropriate systemic antibiotics were prescribed.
DISCUSSION
Although this placebo-controlled study was small, it is the first to demonstrate that topical therapy which produces nitrogen oxides is effective in healing ulcers caused by M. ulcerans infection. After 6 weeks there was evidence that the reduction in the surface areas of ulcers was greater with active therapy than with the placebo. Furthermore, in group II there was a significant reduction in the ulcer surface area during the second 6-week period, when active treatment was administered, whereas there was no significant reduction during the first 6 weeks, when the creams contained no active component. There were differences between the groups in that patients in group I were older, and more had ulcers of longer duration and on the lower limbs, which may have created a bias against a positive outcome. On the other hand, the larger size of ulcers in group I would favor a greater reduction in surface area in that group. However, the accelerated rate of healing in group II during weeks 7 to 12, when active treatment was started, supports the idea that nitrogen oxides promoted healing.
We chose to compare active with placebo treatment during the first 6 weeks and to administer active treatment to both groups thereafter because, when designing the study, we were concerned about the ethics of leaving ulcers untreated longer than 6 weeks. The placebo chosen was an aqueous cream, and the effect of sodium nitrite alone or of citric acid alone was not tested. Although it is possible that these reagents on their own would have an effect on healing, the accompanying paper shows that only the combination caused significant killing of M. ulcerans in vitro (18).
In this study we depended on clinical diagnosis of M. ulcerans infection as the cause of ulcers. An experienced clinician can recognize the features of a painless ulcer with undermined edges and no surrounding cellulitis in a patient who is systemically well. However, lower-limb ulcers are common, and confirmation of the diagnosis would be useful. Such confirmation normally relies on excision biopsy, which is also the conventional treatment; clearly, then, this could not be done. In an attempt to establish the diagnosis by punch biopsies, acid-fast bacilli were identified and cultured for four patients. Cultures of lesions are infrequently positive, and this figure is consistent with that reported by others (9). It may be possible to achieve more positive diagnoses by using a PCR for M. ulcerans DNA, which was not available to us at the time of the study.
Nitrogen oxides are known to be effective in killing a large number of bacteria, fungi, and other microorganisms (10), and generation of nitric oxide by activated macrophages is thought to be important in host defense against Mycobacterium tuberculosis (17). The observation that genetically manipulated mice with inducible nitric oxide synthase deficiency show increased susceptibility to tuberculosis further confirms this hypothesis (5). It has previously been shown that nitric oxide is generated on the skin surface by sequential reduction of sweat nitrate (28), and it has been suggested that nitric oxide may be important in preventing infectious skin diseases. Patients with psoriasis, who are known to release 100- to 1,000-fold-higher levels of nitric oxide on their skin than those of healthy controls, have a lower incidence of bacterial and viral infections (27).
It is now known that acidified nitrite kills M. ulcerans, but there are several mechanisms by which it might do so (18). Nitrite at an acidic pH dismutes to form nitric oxide, which is known to interfere with iron-sulfur clusters and heme groups (7), important in microbial respiration. Acidified nitrite also generates dinitrogen trioxide, a powerful nitrosating agent which will rapidly react with reduced thiols to form nitrosothiols, also thought to be important in microbial killing (8). Nitric oxide will rapidly react with superoxide to form peroxynitrite, a powerful oxidizing and nitrating agent which may be more effective than either of these species in microbial killing (12). Other modes of action of nitric oxide may be involved in the healing of M. ulcerans ulcers, including vasodilatation and augmentation of wound healing. Application of acidified nitrite results in profound vasodilatation (22), and nitric oxide has an important physiological role in normal wound repair (25). Additionally, it may have a function in altering the immune response to infection, since nitric oxide is known to have a number of effects on the immune system.
In this study it is not clear which mechanism is at play in increasing the rate of healing in M. ulcerans disease, but it is possible that this increase results from an interplay of two or more of these factors. The positive effect on ulcer healing was achieved without any significant adverse effects, and the minor pigmentation of lesions that was observed resolved after treatment was finished. This affordable treatment was simple to apply and potentially represents a major advance in the therapy of M. ulcerans disease.
In conclusion, we have shown that a simple topical therapy which generates nitrogen oxides is well tolerated and effective for Buruli ulcer, for which there is no other medical treatment. We do not know the optimum strength of citric acid or sodium nitrite, and we do not know the best frequency or duration of application, but the results generated in this study suggest that further work is warranted to determine these variables in larger randomized studies.
Acknowledgments
We thank Appiah Denkyira, Ashanti regional director of health services, and Nsiah Asare, chief executive of the Komfo Anokye Teaching Hospital, for permitting us to conduct this study. We also thank Beatrice Appau, District Director, and Bernard Fosu, Communicable Disease Officer, Atwima District, for helping to recruit patients; Robert Lartey, the laboratory technician; Doris Ahulu and Doris Kudedze, the research nurses, for rigorous monitoring of patients during this study; and all the patients who participated. Dan Massey gave valuable statistical advice.
This study was supported by a grant from Strakan Pharmaceuticals.
REFERENCES
- 1.Adams, L. B., S. Franzblau, Z. Vavrin, J. B. Hibbs, Jr., and J. L. Krahenbuhl. 1991. l-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 147:1642-1646. [PubMed] [Google Scholar]
- 2.Adjei, O., M. R. W. Evans, and A. Asiedu. 1998. Phenytoin in the treatment of Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 92:108-109. [DOI] [PubMed] [Google Scholar]
- 3.Asiedu, K., and S. Etuaful. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59:1015-1022. [DOI] [PubMed] [Google Scholar]
- 4.Asiedu, K., R. Scherpbier, and M. Raviglione (ed.). 2000. Buruli ulcer: Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland.
- 5.Belaaouaj, A., R. McCarthy, M. Baumann, A. Gao, T. J. Ley, S. N. Abraham, and S. D. Shapiro. 1998. Mice lacking neutrophil elastase reveal impaired host defence against gram negative bacterial sepsis Nat. Med. 4:615-618. [DOI] [PubMed] [Google Scholar]
- 6.Cenci, E., L. Romani, A. Mencacci, R. Spaccapelo, E. Schiaffella, P. Puccetti, and F. Bistoni. 1993. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur. J. Immunol. 23:1034-1038. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, C. E. 1999. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411:290-309. [DOI] [PubMed] [Google Scholar]
- 8.De Groote, M. A., T. Testerman, Y. Xu, G. Stauffer, and F. C. Fang. 1996. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science 272:414-417. [DOI] [PubMed] [Google Scholar]
- 9.Espey, D. K., G. Djomand, I. Diomande, M. Dosso, M. Z. Saki, J. M. Kanga, R. A. Spiegel, B. J. Marston, L. Gorelkin, W. M. Meyers, F. Portaels, M. S. Deming, and C. R. Horsburgh, Jr. 2002. A pilot study of treatment of Buruli ulcer with rifampin and dapsone. Int. J. Infect. Dis. 6:60-65. [DOI] [PubMed] [Google Scholar]
- 10.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grange, J. 1996. Mycobacteria and human disease, 2nd ed. Oxford University Press, New York, N.Y.
- 12.Jones-Carson, J., A. Vazquez-Torres, H. C. van der Heyde, T. Warner, R. D. Wagner, and E. Balish. 1995. γδ T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat. Med. 1:552-557. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan, S. S., J. R. Lancaster, R. E. Basford, and R. L. Simmons. 1996. Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect. Immun. 64:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebanoff, S. J. 1993. Reactive nitrogen intermediates and antimicrobial activity: role of nitrite. Free Radical Biol. Med. 14:351-360. [DOI] [PubMed] [Google Scholar]
- 15.Liew, F. Y., Y. Li, D. Moss, C. Parkinson, M. V. Rogers, and S. Moncada. 1991. Resistance to Leshmania major infection correlates with the induction of nitric oxide in murine macrophages. Eur. J. Immunol. 21:3009-3014. [DOI] [PubMed] [Google Scholar]
- 16.Mancinelli, R. L., and C. P. McKay. 1983. Effects of nitric oxide and nitrogen dioxide on bacterial growth. Appl. Environ. Microbiol. 46:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan, C. 1997. Inducible nitric oxide synthase: what difference does it make? J. Clin. Investig. 10:2417-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips, R., S. Kuijper, N. Benjamin, M. Wansbrough-Jones, M. Wilks, and A. H. J. Kolk. 2004. In vitro killing of Mycobacterium ulcerans by acidified nitrite. Antimicrob. Agents Chemother. 48:3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revill, W. D., R. H. Morrow, M. C. Pike, and J. Ateng. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet 2:873-877. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute. 1996. SAS, version 6.12. SAS Institute, Cary, N.C.
- 21.Thangaraj, H., M. R. W. Evans, and M. H. Wansbrough-Jones. 1999. Mycobacterium ulcerans disease: Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 93:337-340. [DOI] [PubMed] [Google Scholar]
- 22.Tucker, A. T., R. M. Pearson, E. D. Cooke, and N. Benjamin. 1999. Effect of nitric-oxide-generating system on microcirculatory blood flow in skin of patients with severe Raynaud's syndrome: a randomised trial. Lancet 354:1670-1675. [DOI] [PubMed] [Google Scholar]
- 23.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune response in mice lacking inducible nitric oxide synthase. Nat. Med. 375:408-411. [DOI] [PubMed] [Google Scholar]
- 24.Weller, R. 1997. Nitric oxide: a newly discovered chemical transmitter in human skin. Br. J. Dermatol. 137:665-672. [PubMed] [Google Scholar]
- 25.Weller, R. 1999. Nitric oxide, skin growth and differentiation: more questions than answers? Clin. Exp. Dermatol. 24:388-391. [DOI] [PubMed] [Google Scholar]
- 26.Weller, R., A. D. Ormerod, R. P. Hobson, and N. J. Benjamin. 1998. A randomized trial of acidified nitrite cream in the treatment of tinea pedis. J. Am. Acad. Dermatol. 38:559-563. [DOI] [PubMed] [Google Scholar]
- 27.Weller, R., A. Ormerod, and N. Benjamin. 1996. Nitric oxide generation measured directly from psoriatic plaques by chemiluminescence. Br. J. Dermatol. 134:569. [Google Scholar]
- 28.Weller, R., R. Price, A. D. Ormerod, N. Benjamin, and C. Leifert. 2001. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J. Appl. Microbiol. 90:648-652. [DOI] [PubMed] [Google Scholar]