Abstract
Drugs, toxins, and infections are known to cause acute eosinophilic pneumonia. Daptomycin and minocycline are the commonly reported antibiotics associated with acute eosinophilic pneumonia. In this study, we present a case of imipenem/cilastatin-induced acute eosinophilic pneumonia. The patient presented with fever, acute hypoxic respiratory distress, and diffuse ground-glass opacities on the chest CT a day after the initiation of imipenem/cilastatin. Patient also developed peripheral eosinophilia. A reinstitution of imipenem/cilastatin resulted in recurrence of the signs and symptoms. A bronchoscopy with bronchoalveolar lavage showed 780 nucleated cells/mm3 with 15% eosinophil. The patient's clinical condition improved significantly after the discontinuation of imipenem/cilastatin therapy and the treatment with corticosteroid.
Background
Acute eosinophilic pneumonia (AEP) was first described in 1989 and is mostly idiopathic.1 Nevertheless, there has been increasing case reports on offending agents associated with AEP such as drugs, toxins, tobacco smoke and infections. A significant number of antibiotics can cause in pulmonary eosinophilic disease with daptomycin2–5 and minocycline2 3 6 the most frequently reported offending agents. To the best of our knowledge, there is no case report on imipenem/cilastatin-induced pulmonary disorder. In this report, we describe a patient with imipenem/cilastatin-induced AEP.
Case presentation
A 60-year-old woman with a medical history of asthma and diverticulosis who presented with perforated sigmoid colon after an outpatient colonoscopy. She underwent an emergent laparotomy status post segmental colon resection and Hartmann’s colostomy. The faecal appearing peritoneal fluid was sent for analysis. The patient was started empirically on intravenous ampicillin-sulbactam 3 g 6-hourly. The peritoneal fluid culture demonstrated extended-spectrum β-lactamases (ESBLs) Escherichia coli (E. coli) and Klebsiella pneumoniae resistant to ampicillin. She was then switched to imipenem/cilastatin 500 mg 6-hourly at day 4. On day 5, the patient developed fever with a temperature of 101.6°F and acute hypoxic respiratory failure requiring mechanical ventilation. A CT of the chest with intravenous contrast revealed diffuse ground-glass opacities (figure 1). She was treated as acute respiratory distress syndrome (ARDS) following ARDS net protocol. Her white cell count (WCC) on day 5 was 15.6×10/dL without eosinophil.
Figure 1.
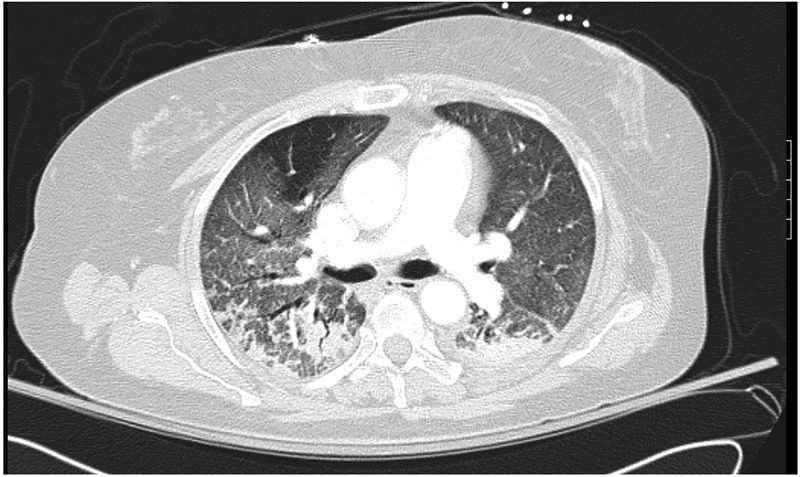
CT of the chest revealed diffuse ground-glass opacities with airspace consolidation a day after the initiation of imipenem/cilastatin.
During the 14th day of antibiotic therapy, she developed peripheral eosinophilia with a peak of 8% and continued to be febrile. A CT of the maxillofacial, chest, abdomen and pelvis were unrevealing of infective sources. Pan-cultures were negative. Further investigations including HIV, hepatitis C, hepatitis B, antinuclear antibodies (ANA), C- and P- antineutrophil cytoplasmic antibody (ANCA) testing were negative. The fever resolved 3 days after the completion of antibiotic therapy. The patient eventually underwent tracheostomy and percutaneous endoscopic gastrostomy tube placement on day 18 of medical intensive care unit (MICU) stay.
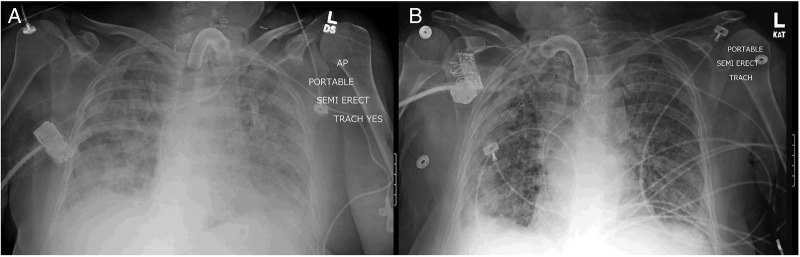
On day 23 of MICU stay, the patient became febrile with a temperature of 100.7 °F. The WCC was 20.6×10/dL. Pan-cultures were sent and a bronchoscopy with bronchoalveolar lavage (BAL) was performed. The BAL specimen showed 3120 nucleated cells/mm3 without eosinophil and Gram-stain revealed Gram-negative rod concerning for ESBL pneumonia. Imipenem/cilastatin was re-initiated. In the next 6 days, the patient continued to be febrile and had persistent leucocytosis with gradually increasing eosinophilia to a peak of 18%. The patient's was switched from pressure support to assist control ventilation due to increased work of breathing. Chest X-ray demonstrated worsening diffuse patchy infiltrates (figure 2A). Imipenem/cilastatin therapy was discontinued given clinical non-response. A second bronchoscopy with BAL was performed and demonstrated 780 nucleated cells/mm3 with a significantly elevated eosinophil percentage of 15%. The silver stain and bacterial, fungal, and mycobacterial cultures were negative. The patient was diagnosed with imipenem/cilastatin-induced AEP.
Figure 2.
(A). Chest X-ray showed diffuse bilateral infiltrates after the reinstitution of imipenem/cilastatin (B). Interval improvement of the bilateral infiltrates after the discontinuation of antibiotics and the initiation of methylprednisolone.
Treatment
The patient was initiated on intravenous methylprednisolone 40 mg 6-hourly with rapid resolution of the fever, leukocytosis and eosinophilia. She was then tapered on methylprednisolone and switched to oral pednisone. A follow-up chest X-ray also demonstrated significant improvement of the diffuse pulmonary infiltrates (figure 2B).
Outcome and follow-up
The patient was discharged to a long-term acute care facility and tracheostomy was successfully decannulated 3 months later. A follow-up CT of the chest 3 months later demonstrated remarkable improvement of the ground-glass opacities (figure 3).
Figure 3.
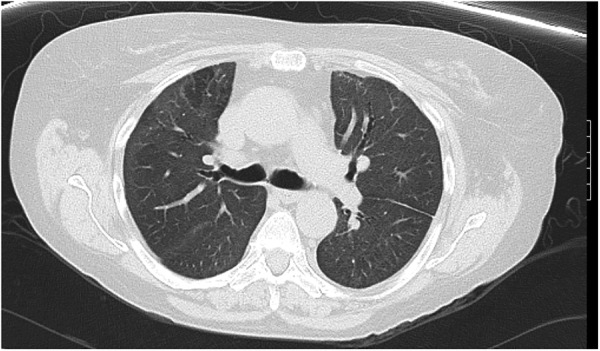
CT of the chest demonstrated improvement of the ground-glass opacities and airspace consolidation 3 months later.
Discussion
AEP is characterised by acute febrile respiratory failure associated with pulmonary infiltrates and pulmonary eosinophilia. The pathogenesis of the AEP is not well understood. Proposed mechanisms include antigenic stimulation of T helper 2 lymphocytes with subsequent productions of interleukin (IL)-5.7 8 IL-5 is thought to be an important cytokine mediator involved in eosinophilic inflammation of the lung in AEP.7–9 AEP is mostly idiopathic, but could be caused by drugs, toxins, tobacco smoke and infections. Daptomycin and minocycline are the most frequently reported offending antimicrobial agents associated with AEP.2–6 To the best of our knowledge, our report is the first to describe a patient with imipenem/cilastatin-induced AEP.
AEP diagnostic criteria is defined by acute febrile respiratory illness (frequently less than a week), hypoxemic respiratory failure (a pulse oxygen saturation of <90% on ambient air or a partial pressure of arterial oxygen (PaO2) of <60 mm Hg), diffuse pulmonary infiltrates on chest radiograph, BAL showing eosinophilia >25% or lung biopsy revealing eosinophilic pneumonia, and the absence of known causes of pulmonary eosinophilic, including drugs, toxins and infections.10
Solomon and Schwarz3 suggests the diagnosis of drug-induced or toxin-induced AEP is made if AEP criteria proposed by Phillips et al is fulfilled, in addition to the presence of potential offending drug or toxin in an appropriate time frame, absence of other causes of pulmonary eosinophilia (parasite and fungal infection), clinical improvement after cessation of the implicated drug or toxin, and recurrence of the pulmonary eosinophilia with rechallenge to the drug or toxin. A rechallenge with the offending agents is not recommended due to its potential harm. The lung biopsy is not routinely needed for the diagnosis of drug or toxin-induced pulmonary eosinophilia.3
Our patient developed acute febrile respiratory failure a day after the administration of imipenem/cilastatin. It was initially thought that the patient developed ARDS secondary to ESBL E. coli peritonitis. The febrile illness resolved after the completion of imipenem/cilastatin. When the imipenem/cilastatin was reinstituted, the patient's symptoms recurred with significantly worsening peripheral eosinophilia. The BAL eosinophil percentage was only 15%. However, there is a clear temporal association between the patient's acute febrile respiratory failure and imipenem/cilastatin use.
The diagnostic criteria of BAL eosinophilia >25% for AEP may not be readily applicable to antibiotic-induced AEP. It occurs in only 64.3% (33.6±18%) and 47.1% (25±17%) of daptomycin-induced and minocycline-induced AEP, respectively (table 1).5 6 In the study by J Phillips et al,5 a new daptomycin-induced AEP diagnostic criteria was recommended to substitute the criteria of BAL eosinophilia >25% to an abnormal BAL esosinophilia percentage in accordance to the institutions’ standards.
Table 1.
Summary of the present case, and cases of daptomycin-induced and minocycline-induced AEP in literature
| Antimicrobial agents | Reported cases of antimicrobial-induced AEP, n | Peripheral eosinophilia, n (%) | BAL eosinophil percentage >25%, n (%) | BAL eosinophil percentage, mean±SD | Duration of antimicrobial therapy at symptom onset ≤1 week, n (%) | Corticosteroid use, n (%) |
|---|---|---|---|---|---|---|
| Imipenem/cilastatin | 1 | 1 (100) | 0 (0) | 15% | 1 (100) | 1 (100) |
| Daptomycin | 24 | 13 (54.2) | 9 of 14 (64.3) Non-specific percentage in 2 cases, ND/NR in 8 cases |
33.6±18% | 3 (12.5) | 17 (71) |
| Minocycline | 24 | 19 (79.2) | 8 of 17 (47.1) Non-specific percentage in 1 case, ND/NR in 6 cases |
25±17% | 8 (33.3) | 12 (50) |
AEP, acute eosinophilic pneumonia; BAL, bronchoalveolar lavage; ND, not done; NR, not reported.
Idiopathic AEP typically lacks peripheral eosinophilia.11 Our literature review demonstrated peripheral eosinophilia is associated with antimicrobial agent-induced AEP. Peripheral eosinophil was found in 54.2% daptomycin-induced and 79.2% of minocycline-induced AEP (table 1).5 6 The present case demonstrated peripheral eosinophilia by both absolute count and percentage.
Learning points.
Antimicrobial agent-induced acute eosinophilic pneumonia (AEP) is uncommon and may be hard to be distinguished from acute respiratory distress syndrome.
Imipenem/cilastatin can cause AEP and should be suspected in the right clinical setting especially in patient who develop acute febrile respiratory illness with radiographic evidence of diffuse pulmonary infiltrate and peripheral eosinophilia.
Timely diagnosis and appropriate treatment including the removal of suspected antimicrobial agent and the initiation of intravenous corticosteroid are essential to prevent potential fatal complications.
Patient should also be avoided from future exposure to the offending agent.
Acknowledgments
The authors appreciate the service of Infectious Diseases division at Crozer Chester Medical Center, in particular Dr Gustavo Vasquez in collaboration on the diagnostic process.
Footnotes
Contributors: KSF, MP and WB were involved in the care of the described patient. AL was involved in the critical analysis of the data and conception of the manuscript. KSF coordinated the creation of the final manuscript and was responsible for the submission. All the authors critically reviewed the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Allen JN, Pacht ER, Gadek JE et al. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med 1989;321:569–74. 10.1056/NEJM198908313210903 [DOI] [PubMed] [Google Scholar]
- 2.The Drug-Induced Respiratory Disease Website; Department of Pulmonary Medicine and Intensive Care University Hospital Dijon France 2012. http://www.pneumotox.com (accessed 10 Sep 2015).
- 3.Solomon J, Schwarz M. Drug-, toxin-, and radiation therapy-induced eosinophilic pneumonia. Semin Respir Crit Care Med 2006;27:192–7. 10.1055/s-2006-939522 [DOI] [PubMed] [Google Scholar]
- 4.Miller BA, Gray A, Leblanc TW et al. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010;50:e63–8. 10.1086/652656 [DOI] [PubMed] [Google Scholar]
- 5.Phillips J, Cardile AP, Patterson TF et al. Daptomycin-induced acute eosinophilic pneumonia: analysis of the current data and illustrative case reports. Scand J Infect Dis 2013;45:804–8. 10.3109/00365548.2013.805427 [DOI] [PubMed] [Google Scholar]
- 6.Hung SW. Minocycline-induced acute eosinophilic pneumonia: a case report and review of the literature. Respir Med Case Rep 2015;15:110–14. 10.1016/j.rmcr.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med 2006;27:142–7. 10.1055/s-2006-939517 [DOI] [PubMed] [Google Scholar]
- 8.Allen JN. Drug-induced eosinophilic lung disease. Clin Chest Med 2004;25:77–88. 10.1016/S0272-5231(03)00141-2 [DOI] [PubMed] [Google Scholar]
- 9.Nishigaki Y, Fujiuchi S, Yamazaki Y et al. Increased vascular endothelial growth factor in acute eosinophilic pneumonia. Eur Respir J 2003;21:774–8. 10.1183/09031936.03.00085903 [DOI] [PubMed] [Google Scholar]
- 10.Philit F, Etienne-Mastroïanni B, Parrot A et al. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med 2002;166:1235–9. 10.1164/rccm.2112056 [DOI] [PubMed] [Google Scholar]
- 11.Pope-Harman AL, Davis WB, Allen ED et al. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine (Baltimore). 1996;75:334–42. 10.1097/00005792-199611000-00004 [DOI] [PubMed] [Google Scholar]