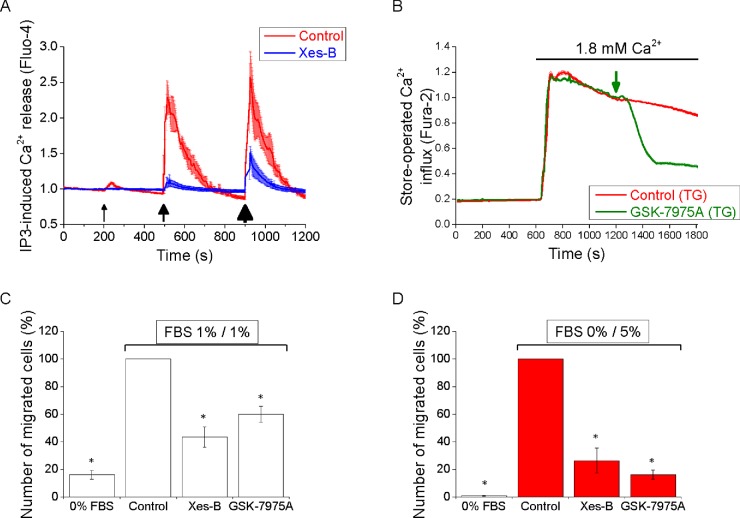
Figure 5. Inhibition of Ca2+ signalling complexes suppresses PANC-1 cell migration.
(A) Xestospongin-B (Xes-B) blocks IP3-induced Ca2+ release in pancreatic adenocarcinoma cells. Ca2+ releases from internal stores were measured in cells loaded with caged IP3 in the absence (control; red trace, 28 cells) or presence of 50 μM xestospongin B (blue trace, 29 cells). The traces were composed of normalized Fluo-4 fluorescence measurements (mean values ± S.E.M. for individual time points). Pulses of UV light induced the IP3 uncaging (i.e. release of IP3 from its caged precursor) and subsequent releases of Ca2+ from internal stores into the cytosol. The intensities of the black arrows indicate the duration of uncaging (3, 8 and 20 s). In these experiments nominally Ca2+-free extracellular solution was used to reveal cytosolic Ca2+ responses occurring specifically due to Ca2+ release from the intracellular stores. (B) GSK-7975A inhibits SOCE in PANC-1 cells. Cells were pre-treated with TG for approximately 20 min before the beginning of the experiments in order to permanently deplete the ER Ca2+ stores, and maintained in nominally Ca2+-free solution. Increase in extracellular [Ca2+] to 1.8 mM resulted in Ca2+ influx via the SOCE pathway. Red trace shows control experiment (192 cells). Green trace (151 cells) shows significant inhibition of SOCE by 30 μM GSK-7975A. The green arrow indicates the moment of GSK-7975A addition. The traces were composed from normalized mean values ± S.E.M. for individual time points of Fura-2 fluorescence ratio (F340/F380) measurements. (C and D) Xestospongin-B and GSK-7975A suppress migration of PANC-1 cells. PANC-1 cells were subjected to both symmetric (1% FBS in both chambers) (C) and asymmetric (0% FBS in the upper chamber and 5% FBS in the lower chamber) (D) Boyden chamber migration assay for 6 h at 37°C in the absence (Control) or presence of the inhibitors. 0% FBS conditions (in both chambers, leftmost bars on both panels) were employed for comparison (this effectively inhibits cell migration). The number of migrated cells in each inhibitor-treated group was normalized to that in the control group for every individual experiment. Means ± S.E.M. were obtained from at least three independent experiments. In all cases the number of cells migrated in the presence of an inhibitor was statistically different from that in the control conditions.