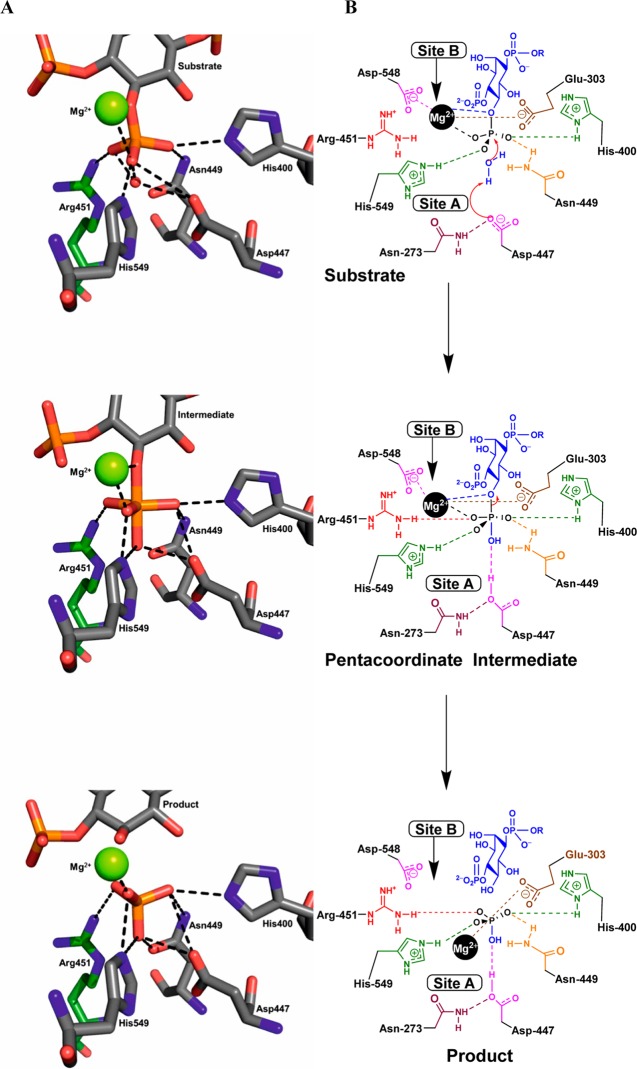
Figure 8.
(A) Stick diagram showing participants in a pentacoordinate intermediate based on the AP endonuclease mechanism. After nucleophilic attack of an activated water molecule the trigonal bipyramidal intermediate is stabilized by a series of residues and the active site Mg2+ ion. Arg-451 (colored green) was not originally suggested as part of the mechanism, but is located such that it may help stabilize the intermediate and is conserved in all 5-phosphatases. Only schematic involvement of the various residues is depicted. (B) Mechanism for the hydrolysis of the 5-phosphate of Ins(1,4,5)P3 by INPP5B, based upon the stick diagram and interactions found in (A) together with additional contributing amino acid residues and the movement of the Mg2+ ion from site B towards site A. Attack by a water molecule produces a trigonal bipyramidal intermediate that collapses releasing the phosphate anion. The amino acids are color-coded for easier recognition. The anion that forms at the 5-position is probably quenched with a proton originating from a nearby water molecule, possibly Mg2+-bound, or from the adjacent protonated 4-phosphate group. This has not been shown on the diagram for the sake of clarity. Key: R = H for Ins(1,4,5)P3, R = diacylglycerol for PtdIns(4,5)P2. Only schematic involvement of the various residues is depicted. Negative charges during phosphoryl transfer are not shown.