Figure 4.
Hinge Dimerization and Head Engagement Control the Conformation of Smc/ScpAB
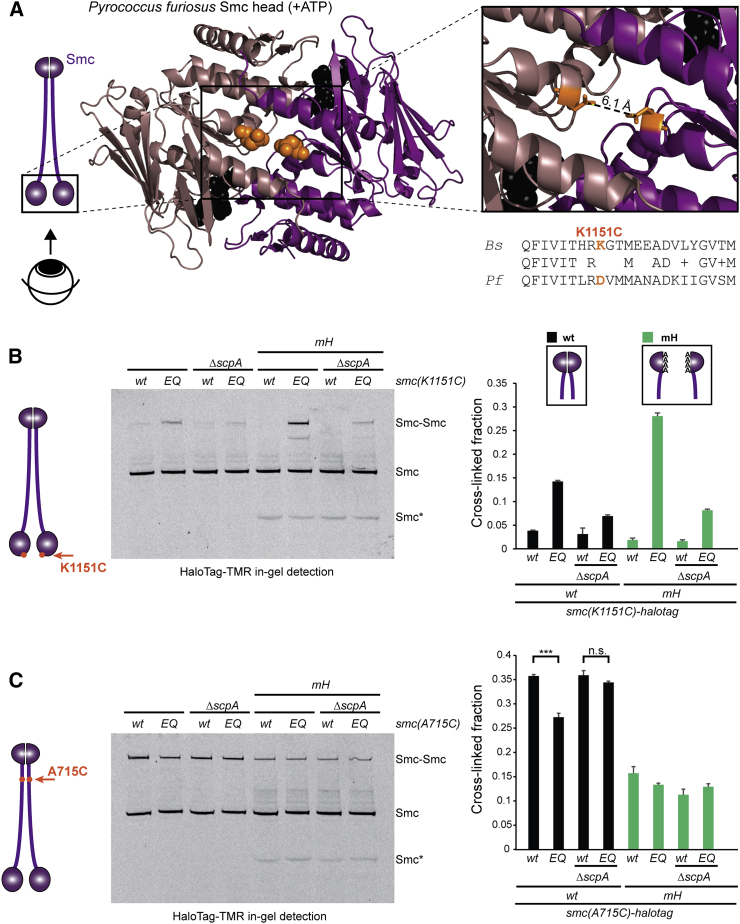
(A) Structure of ATP engaged Pf Smc head domains (PDB: 1XEX) in brown and pink colors, respectively, (bottom view). Residue D1131 is indicated in ball representation in orange colors (middle panel). The distance between the carboxyl carbon atom in the side chains of the D1131 symmetry mates is estimated to be ∼6 Å (right panel). A sequence alignment between PfSmc and BsSmc shows that K1151 in BsSmc corresponds to D1131 in PfSmc.
(B) In vivo BMOE crosslinking of Smc(K1151C)-HaloTag in cells of strains BSG1488, BSG1509, BSG1512, BSG1547, BSG1597–BSG1598, BSG1791, and BSG1800. Four endogenous cysteine residues were replaced by serines. Cross-linked Smc-Halotag species were detected by in-gel fluorescence of the HaloTag-TMR substrate (left panel). Smc∗ indicates a degradation product of Smc(mH). The graph (right panel) shows mean values and SDs from three replicates.
(C) Same as in (B) using A715C as sensor cysteine for formation of Smc rods by the hinge proximal Smc coiled coil. In vivo crosslinking of Smc(A715C) with bismaleimidoethane (BMOE) in strains BSG1921–BSG1924, BSG1949–BSG1951, and BSG2036. T test statistics: ∗∗∗p ≤ 0.001; not significant (n.s.), p > 0.05.
See also Figure S4.