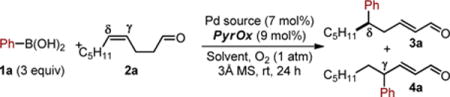
Table 1.
Optimization of the enantioselective relay Heck reaction of 2a.
| |||||
|---|---|---|---|---|---|
| Entry | Pd source | Solvent | yield of 3a (%)a | er (3a)b | δ:γc |
| 1 | Pd(MeCN)2(OTs)2 | DMF | 73 | 98:2 | 5.8 |
| 2 | None | DMF | – | – | – |
| 3 | Pd(MeCN)2(OTs)2d | DMF | trace | – | – |
| 4 | Pd(MeCN)2(OTs)2e | DMF | trace | – | – |
| 5 | Pd(MeCN)2(OTs)2f | DMF | 65 | 98:2 | 5.2 |
| 6 | Pd(MeCN)2(OTs)2 | DMA | 78 | 99:1 | 15 |
| 7 | Pd(MeCN)2(OTs)2 | 1,4-dioxane | 73 | 98:2 | 7.8 |
| 8 | Pd(MeCN)2(OTs)2 | THF | 38 | 98:2 | 9.3 |
Conditions: 1a (0.75 mmol, 3.0 eq), 2a (0.25 mmol, 1.0 eq), [Pd] (0.01875 mmol, 7.5 mol%), ligand (0.0225 mmol, 9 mol%), solvent (3 mL), O2 (1 atm), rt, 24 h.
Reported yields are of isolated 3a as a >20:1 ratio of the E-alkene isomer.
Determined by SFC equipped with a chiral stationary phase.
Determined by NMR.
In the absence of 3Å MS.
In the absence of ligand.
Using air (1 atm). DMF = N,N-dimethylformamide; DMA = N,N-dimethylacetamide, THF = tetrahydrofuran.
