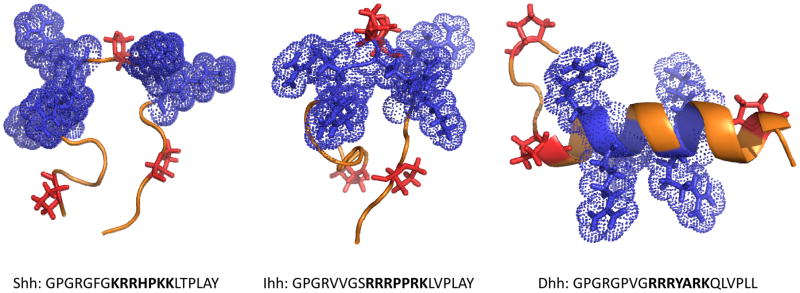
Figure 2.
Structure of CW motifs in Shh, Ihh and Dhh. Secondary structure predications were carried out using the I-TASSER server (45) and resulting structures were visualized using PyMol. The peptides are oriented with the N-terminus on the left; side chains of basic residues within the CW motifs are in blue while the side groups of proline are in red. Note that CWs of Shh and Ihh have a largely random coil configuration with central kinks due to the proline residue(s) while the CW of Dhh exhibits a more helical configuration. The sequences examined are presented below each structure; the CW motif is in bold type and flanking residues are in plain typeset.