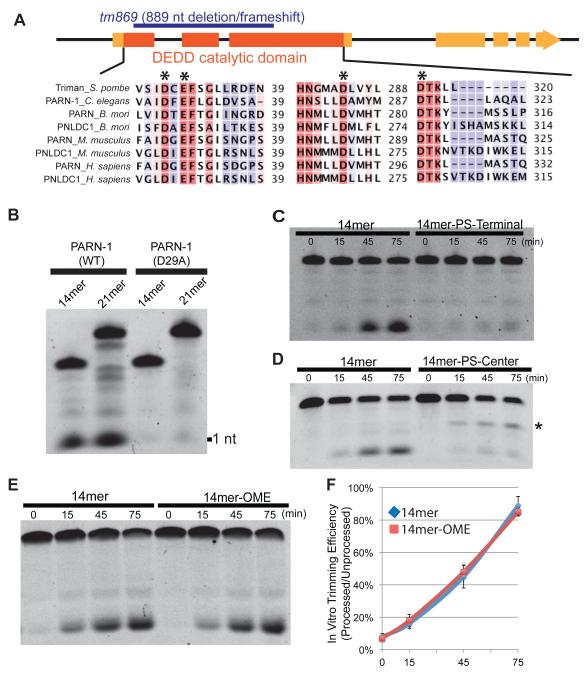
Figure 4. Recombinant PARN-1 possesses 3′-to-5′ exonuclease activity in vitro.
(A) Schematic of the parn-1 genes showing exons (boxes) and intron (lines) with the catalytic ribonuclease domain shaded in dark orange. The deletion allele (tm869) is highlighted in blue. The alignment shows human, mouse, C. elegans, B. mori, and S. pombe PARN and poly(A)-specific ribonuclease-like domain containing (PNLDC) protein with conserved catalytic residues (asterisk).
(B) Electrophoresis analysis on 21nt and 14nt 5′ Fluorescein labeled RNA substrates incubated with purified PARN-1 (WT and D29A) proteins.
(C-E) Electrophoresis analysis of 14-mer RNAs containing 3′ hydroxyl (14mer) and phosphorothioate (PS) bonds from positions 10 to 14 (14mer-PS-Terminal) (C), 14-mer RNAs with PS bond between positions 7 to 8 (14mer-PS-Center). Asterisk represents the degradation intermediate (D), and 14-mers containing 3′ hydroxyl and 2′–O–methylated 3′ termini (14mer-OME) (E).
(F) Quantification of the ratio between processed and unprocessed products among biological replicates. The average of six replicates for 14mer and the average of three replicates for 14mer-OME are shown. Error bars represent standard deviation.
See also Figure S4.