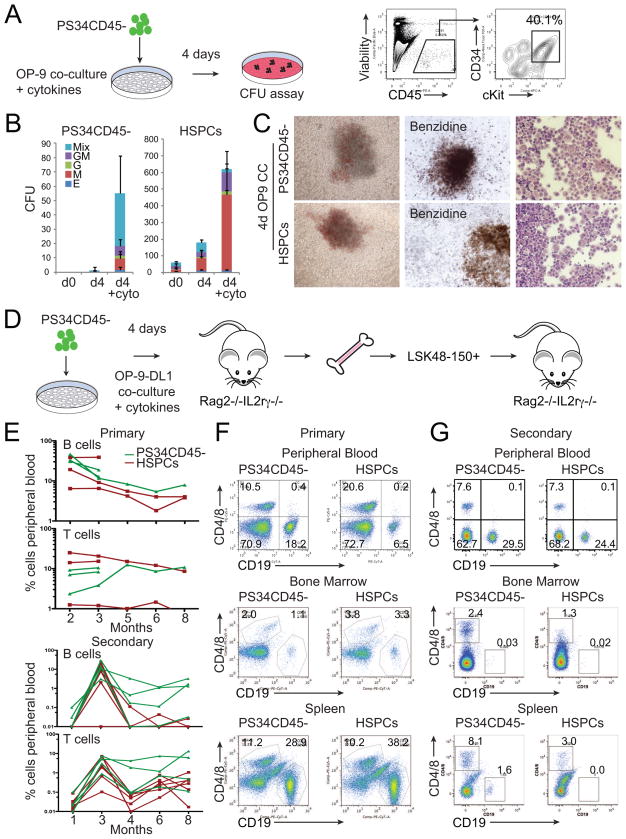
Figure 7. Placental Hemogenic Cells Engraft Immunodeficient Mice after OP9-DL1 Co-culture.
(A) E12.5 PS34CD45− cells were co-cultured on OP-9 in the presence of cytokines (SCF, Fltl3L, IL-3, IL-6 and TPO) for 4 days. FACS analysis shows the generation of CD45+CD34+cKit+ cells that were tested for clonogenic activity.
(B) Number and type of colonies generated at day 0, and after co-culture on OP9 of PS34CD45− or HSPCs (CD45+cKit+CD34+) with or without cytokines (mean ± SD, n=3).
(C) Pictures of representative mixed colonies (left), Benzidine hemoglobin staining (middle) and modified Giemsa staining (right).
(D) E12.5 placentas were dissociated and PS34CD45− cells isolated and cultured in gelatin-coated dishes or co-cultured with OP-9 or OP9-DL1 cells in the presence of cytokines for 4 days. Co-cultures were dissociated and transplanted intravenously into Rag2−/−/IL2Rγc−/− mice.
(E) Mice were bled from 2–8 (experiment 1) or 2–3 (experiment 2) months after transplantation (upper panel) with PS34CD45− co-cultured with OP9-DL1 (green lines). Additional mice were transplanted with freshly isolated HSPCs (red lines). After 14 weeks or 8 months of primary transplant, engrafted mice were sacrificed, and HSCs (LSK48-150+) were purified from bone marrow and transplanted intravenously into secondary Rag2−/−/IL2Rγc−/− mice (lower panel).
(F) After 12–14 weeks the presence of B (CD19+) and T (CD4+/CD8+) cells in peripheral blood, bone marrow and spleen was analyzed by flow cytometry.
(G) Analysis for the presence of B and T cells in peripheral blood after 3 months and in spleen and bone marrow after 8 months of secondary transplantation.