Figure 1.
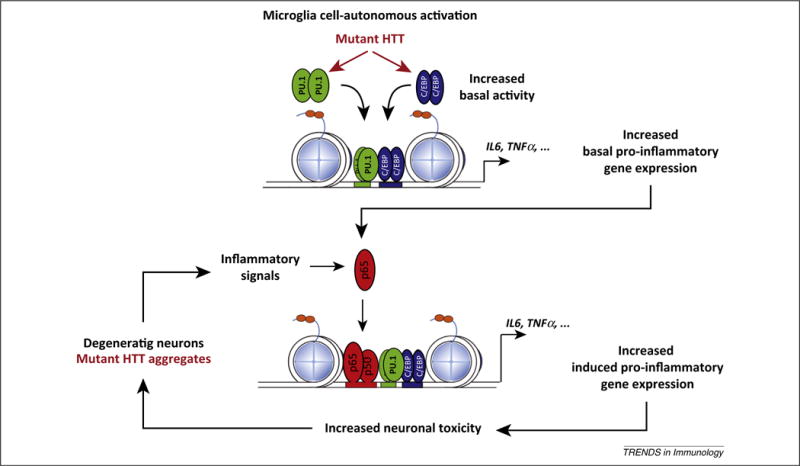
A model for mutant Huntingtin (HTT) microglia cell-autonomous activation and reactive microglia responses to neurodegeneration. In the presence of mutant HTT, increasing PU.1 expression and PU.1- CCAAT/enhancer-binding protein (C/EBP) promoter binding leads to increased enhancer activity under basal conditions that results in increased expression of basal pro-inflammatory and neurotoxic genes. This phenomenon increases the sensitivity to pro-inflammatory signals. In fact, under conditions of sterile inflammation, mutant HTT-expressing microglia appear to be more efficient in inducing neuronal death. We hypothesize that components of dead neurons or mutant HTT aggregates could trigger sterile inflammation, and this, in turn, could lead to further microglia activation, resulting in increased neuronal death and the activation of a chronic ‘feed-forward loop’. Adapted from [34]. Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
