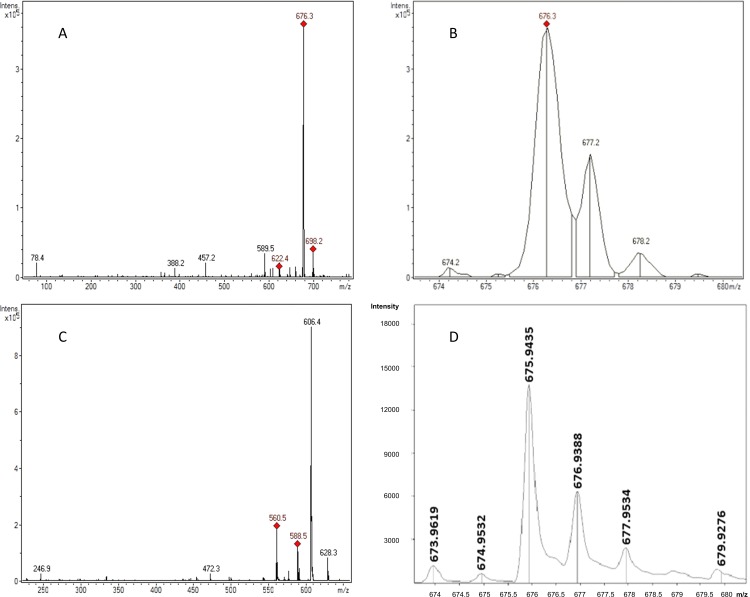
Fig 7. Mass spectrometry of the ferrisiderophore complex.
A-C, Siderophore iron complex determined by ESI-MS. A, The molecular peak of the ferrisiderophore complex has a mass of 676.3 Da [M(Fe)+H]+. The peak of the siderophore without iron has a molecular mass of 622.4 Da [M)+H]+. The peak of 589.5 Da is the fragmentation product [M(Fe)+H-putrescine]+. The peak of 698.2 Da is a Na adduct [M(Fe)+Na]+. B, Isotopic distribution pattern of the quasimolecular ion of 676.3 Da. The peak of 676.3 Da is the ferrisiderophore complex with most abundant isotopes. The peaks of 674.2 Da, 677.2 Da and 678.2 Da are isotope peaks. C, Fragments of the siderophore iron complex. The peak of 606.4 Da is the fragmentation product formed mainly by the elimination through a McLafferty rearrangement of CH2CHCH2CH2NH2. The peaks of 588.5 Da and 560.5 Da are other fragmentation products bound to iron. D, Isotopic distribution pattern of the ferrisiderophore determined by MALDI-TOF mass spectrometry. The peak of 675.9435 Da corresponds to a population of ferrsiderophore complex that contains most abundant carbon12, hydrogen1, nitrogen14, oxygen16 and iron56 isotopes. The peaks of 676.9388 Da, 677.9534 and 679.9276 Da correspond to populations of complexes that contain lower abundance isotopes in their structure. The peak of 673.9619 Da and 674.9532 Da corresponds to a population of complexes that contain the isotope iron54.