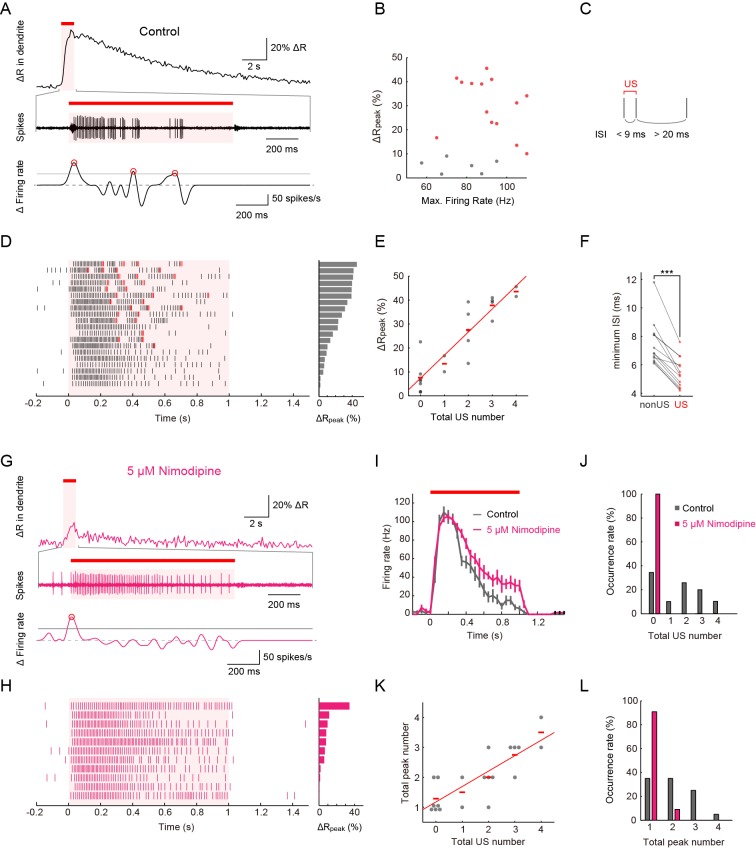
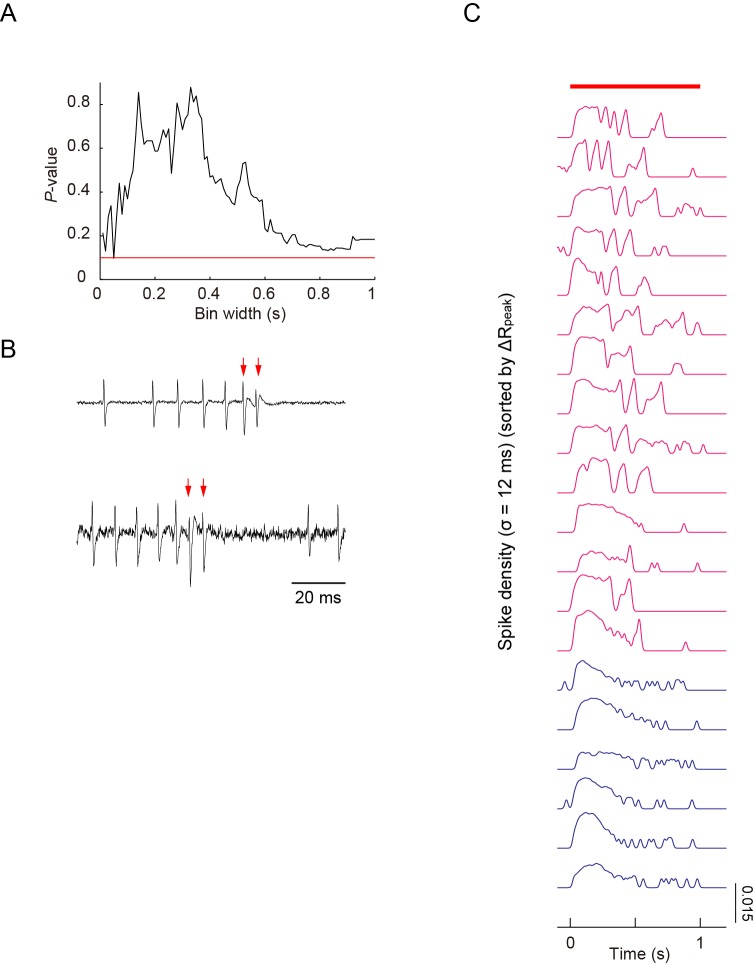
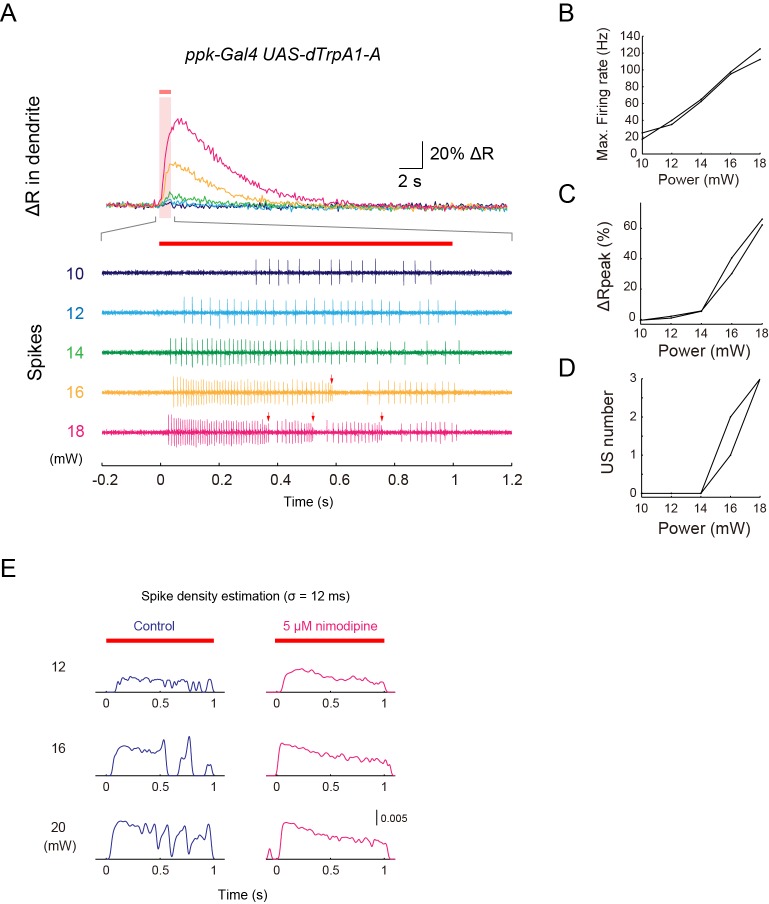
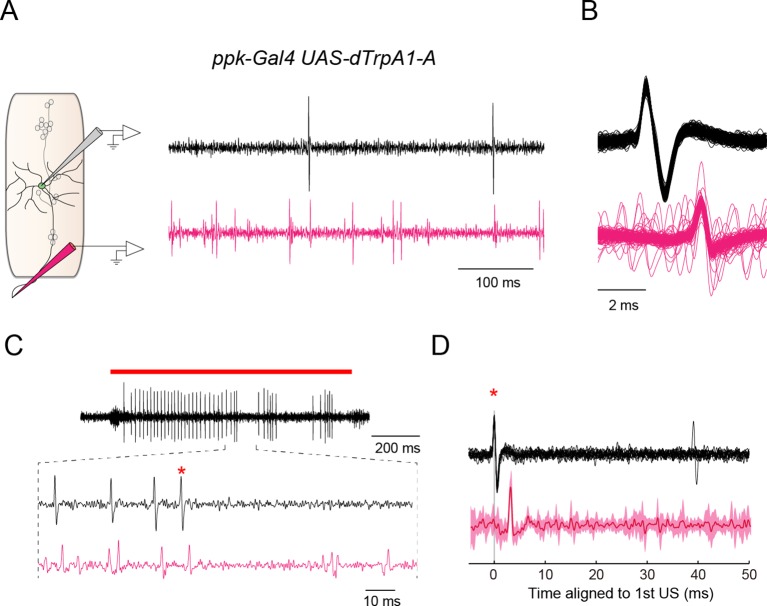
Figure 3. Simultaneous recordings of firing responses and dendritic Ca2+ transients in fillet preparations of larvae upon noxious thermal stimulation.
Data obtained from fillet preparations of control larvae (A–F), those from L-type VGCC-blocked larva (G and H), and their comparisons (I–L). In each trial, 40-mW IR laser was focused onto a proximal dendritic branch for 1 s (indicated by red bars above traces, and magenta shadings in (A, D, G, I and H) and the Ca2+ transient was detected from a ROI that was set on a distal branch 100 μm away from the focus of the IR-laser beam. (A and G) Representative recordings of a control larva (A) and a larva treated with 5 μM Nimodipine (G). Dendritic Ca2+ fluctuations for 20 s (top) and an enlarged trace of an extracellular recording for 1.675 s including the 1 s duration of the IR-laser irradiation (middle). The time derivative of firing rate fluctuation based on the spike density estimation with the Gaussian kernel (σ = 25 ms) and the normalized threshold settings (bottom). The peak of firing rate fluctuation is defined as in the text and marked with red circles. (B) Peak amplitudes of Ca2+ transients are plotted against maximum firing rates (defined in Materials and methods). ΔRpeak is defined as the averaged ratio of 400–700 ms after the cessation of IR-laser stimulation, which is employed as a representative value of each trial. See also the legend of D. A significant correlation was not observed (p > 0.46, ρ = 0.17; Spearman's rank correlation test; n = 20 cells). (C) The definition of 'unconventional spikes' (US). Signatures of US were extracted according to the following two conditions: (1) the first interspike interval (ISI) of three sequential spikes was less than 9 ms, and (2) the second ISI was longer than 20 ms. Then, a pair of the first and second spikes within each sorted triplet was designated as 'US'. (D and H) Raster plots of firing (left) and magnitudes of ΔRpeak (right). (D) All trials in panel (B), where control larvae were used, are sorted in a descending order of magnitude of ΔRpeak (right gray bars). Red raster lines indicate US. Six trials that elicited no US are labeled by gray color in B. (H) When fillet preparations were treated with 5 μM Nimodipine, none of the 11 trials elicited US during IR irradiation. (E) Amplitudes of ΔRpeak are plotted against total US numbers for each trial. Short horizontal red bars indicate the averages of ΔRpeak, and the red line is a linear regression of plotted data (p < 1.9 × 10–8, ρ = 0.91; Spearman’s rank correlation test; n = 20 cells). Our original definition of US in fact displayed a suboptimal correlation to the peak amplitude. We redefined USs using different parameter sets (lengths of the first and second interspike intervals) and calculated Spearman’s correlation coefficients to estimate how well the total US number is correlated with the peak amplitude. As a result, our original definition in C in fact displayed a suboptimal correlation to the peak amplitude (data not shown). (F) Comparative analysis of ISI between US and between non-US events. For trials with at least one US, the minimum length of ISI between US in each trial was compared to that between non-US (p < 5.2 × 10–6; paired t-test; n = 13 cells). (I–L) Temporal patterns of firing rates (I) and histograms of the total US number (J) or the total peak number (L) in control and Nimodipine-treated larva (gray and magenta, respectively). See also the legend of A. (K) A plot of total peak numbers versus total US numbers for each trial in the control. Data in I are presented as mean ± s.e.m.