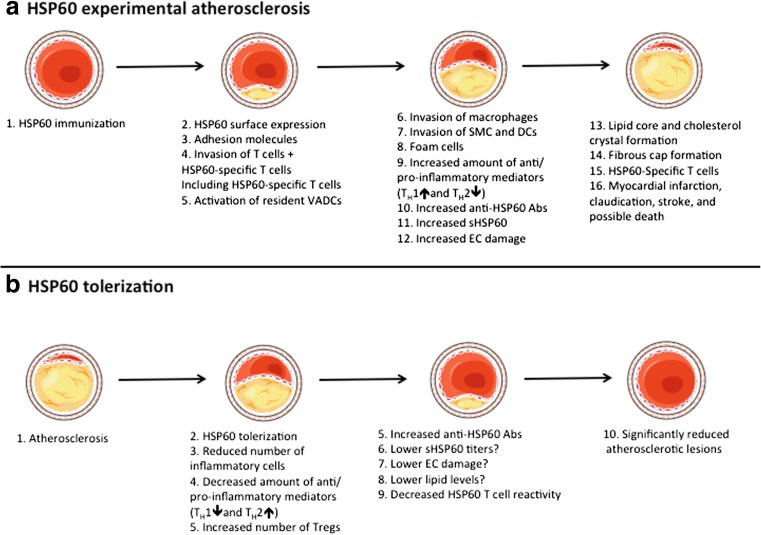
Fig. 1.
a Under physiological conditions, vascular endothelial cells (ECs) do not express heat shock protein (HSP)60 on the surface; however, after HSP60/65 immunizations (or other kind of stressors), HSP60 is transported and appears on the EC cell surface. The surfaces expression of HSP60 appears simultaneously with the expression of adhesion molecules. Activated T cells are the first invaders of the arterial intima in early atherosclerotic lesions. Early, still inapparent, atherosclerotic lesions show HSP60-specific T cells. Pre-existing resident vascular-associated dendritic cells (VADCs) might present the HSP60 antigen, either locally in the intima or after transport to draining lymph nodes. An increased number of macrophages, smooth muscle cells (SMCs), lipid deposition, foam cells formation, and release of pro-inflammatory mediators both locally and into the circulation are seen in the more developed plaque. Increased titers of anti-HSP60 autoantibodies and soluble HSP60 (sHSP60) are detected in the circulation. Stressed, but not unstressed ECs can be lysed by anti-HSP60 anti-HSP60 antibodies in a complement-mediated fashion or via antibody-dependent cellular cytotoxicity. Also, late complicated plaques show HSP60-specific T cells. Some of these epitopes are shared in early vs. late lesions; however, some only exist in each subset. If exposure of stress persists, the plaque becomes more complex and forms a core of necrotic and apoptotic cells, cell debris, and cholesterol crystals, along with a fibrous cap. Rupture of unstable plaques exposes the core and can lead to thrombus formation, myocardical infarction, claudication, stroke, and death. b After tolerization with full-length HSP60/65 or preferable with their peptide(s), a lower number of lesional T cells, macrophages, and SMCs are seen. A reduced level of TH1 and increased level of TH2 mediators can be found locally, in secondary lymphoid organs, and/or in the circulation. An increased number and suppressive capacity of regulatory T cells has also been found. Moreover, increased anti-HSP60 IgG1 (auto)antibodies are found in the circulation after HSP60 treatment, which may lead to a lower titer of sHSP60 and decreased EC damage. The lipid reduction that has been found in tolerized animals is probably a by-product of HSP60/65 immune interference and not a consequence of the tolerance. However, it is still not yet fully elucidated if lipid levels can be reduced after HSP60/65 tolerization. A decreased T cell reactivity in the secondary lymphoid organs against HSP60 antigens indicates an induction of tolerance to HSP60. Partly adapted from Servier Medical Art