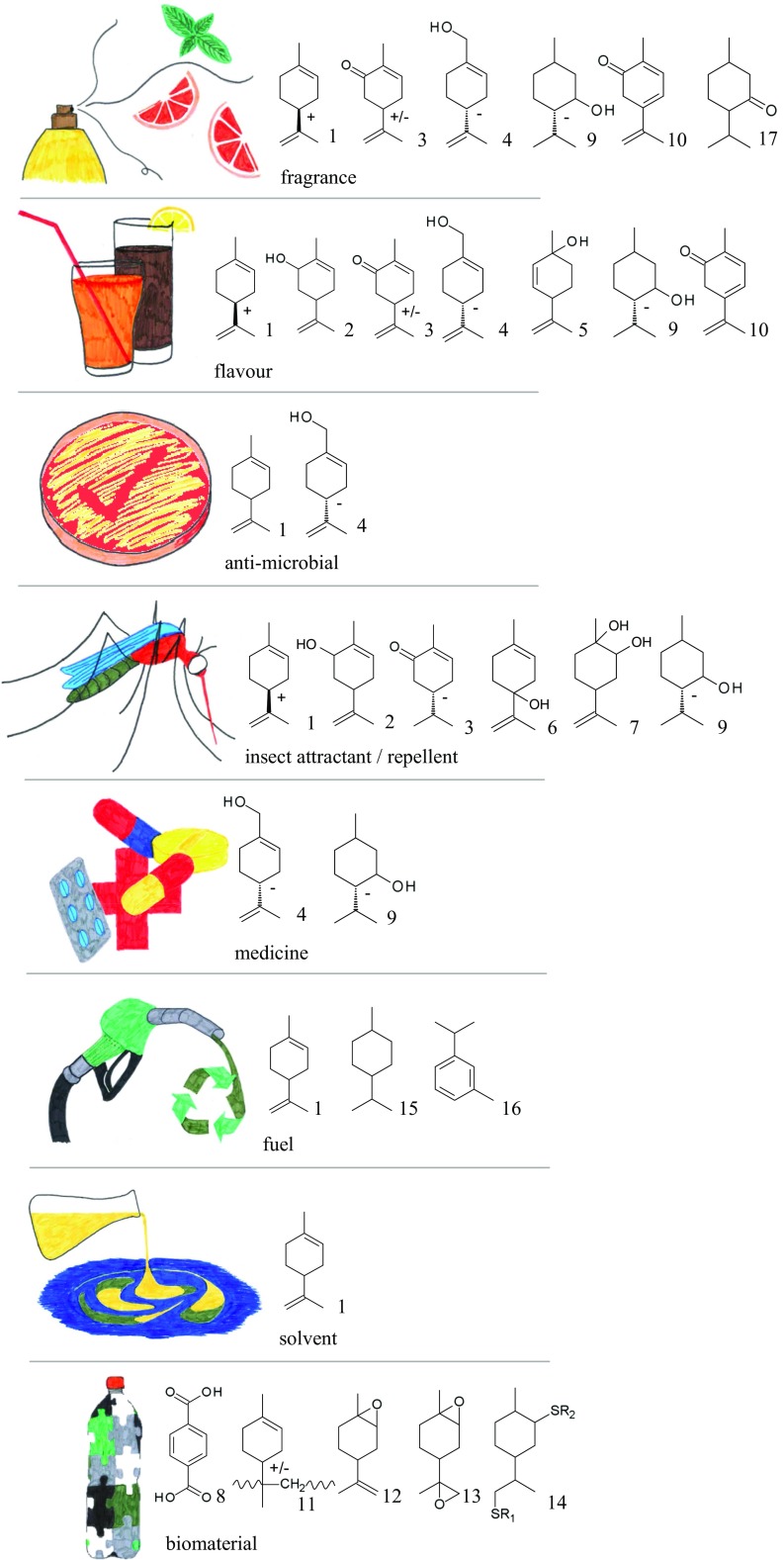
Fig. 1.
Applications of limonene and limonene-derived molecules made by plants (1, 2, 3, 4, 8, 9, 10), microbes (1, 2, 3, 4, 5, 6, 7, 8, 12), and/or chemical synthesis (1, 3, 11, 12, 13, 14, 15, 16, 17) (Duetz et al. 2003; Lerin et al. 2010; Duetz et al. 2001; Bowen 1975; Weldon et al. 2011, Tripathi et al. 2009, Lange 2015, Colonna 2011, Ciminno 1998, Ciriminna 2014, Firdaus 2011, Tracy 2009). 1 Limonene; 2 carveol; 3 carvone; 4 perillyl alcohol; 5 p-mentha-2,8-diene-1-ol; 6 p-mentha-1,8-dien-4-ol; 7 p-menth-8-ene-1,2-diol; 8 terephthalic acid; 9 menthol; 10 dehydrocarvone; 11 polylimonene; 12 limonene monoepoxide; 13 limonene di-epoxide; 14 product of thiol di-addition (R1 and R2 are thiol-side groups, e.g., 2-mercaptoethanol, methyl thioglycolate, or thioglycerol); 15 1-isopropyl-4-methylcyclohexane; 16 m-cymene; 17 menthone; + and – indicate where a single enantiomer is used; +/− means either enantiomer can be used, but not as a mixture