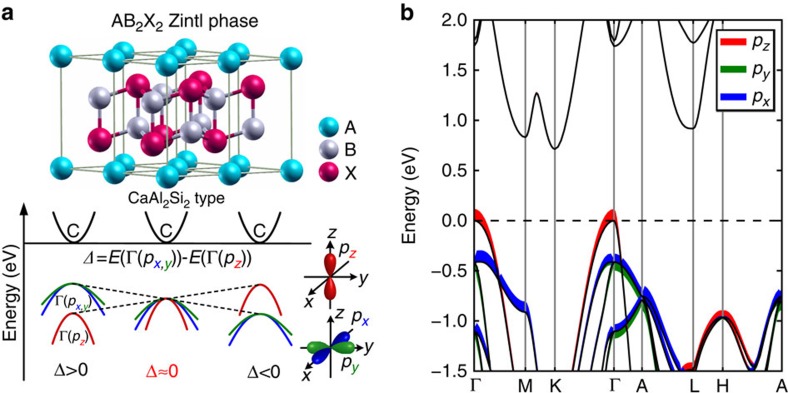
Figure 1. Orbital engineering to realize threefold degenerate p orbitals in layered CaAl2Si2-type Zintl compounds.
(a) Crystal structure and electronic bands of CaAl2Si2-type Zintl compounds. Nondegenerate band Γ(pz) and doubly degenerate band Γ(px,y) are mainly composed of pz and px,y orbitals from anions, respectively. Δ is the crystal field splitting energy between px,y and pz orbitals at the Γ point. (b) Orbital-projected band structure of representative CaAl2Si2-type Zintl compound Mg3Sb2 with negative Δ value. px, py and pz orbitals of Sb anions are projected on the band structure. Curve width indicates the relative weight of the component. Two representative compounds Mg3Sb2 (Δ<0) and SrZn2Sb2 (Δ>0, Supplementary Fig. 2) are used to demonstrate the p orbital characteristics of CaAl2Si2-type Zintl materials.