FIGURE 2.
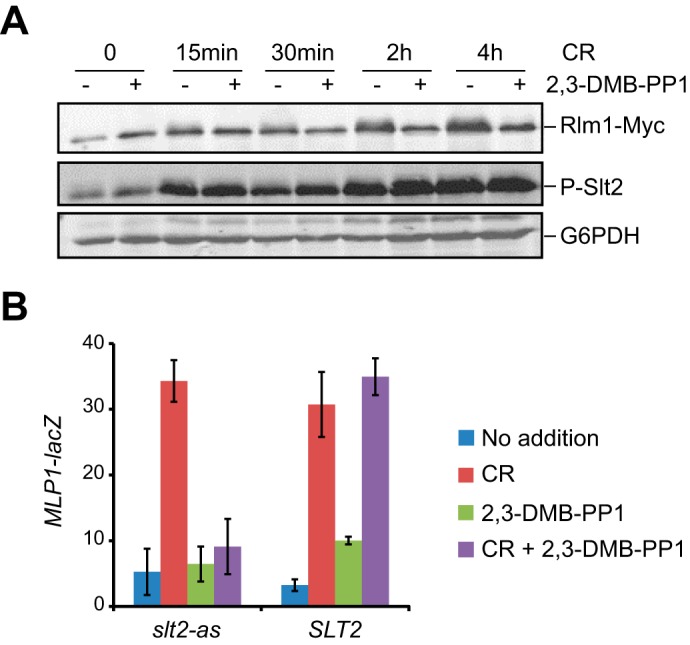
Rlm1 activation is inhibited by 2,3-DMB-PP1 in Slt2-as-expressing cells. A, Western blot analysis of extracts of YMM1 (slt2Δ RLM1::6MYC) cells transformed with pRS316-slt2-as treated with 30 μg/ml of Congo red for the indicated times in the presence (+) or absence (-) of 20 μm 2,3-DMB-PP1. Rlm1-Myc, phosphorylated Slt2, and G6PDH (as loading control) were detected with anti-Myc, anti-phospho-p44/42, and anti-G6PDH antibodies, respectively. B, β-galactosidase activity of cell extracts from Y00993 (slt2Δ) cells bearing pMLP1-LacZ and pRS316-SLT2 or pRS316-slt2-as. Cells were left untreated or treated for 4 h with 30 μg/ml Congo red and/or 20 μm 2,3-DMB-PP1 as indicated in the graphic. Data shown represent the average of β-galactosidase activity of three independent transformants. Error bars indicate S.D.
