FIGURE 1.
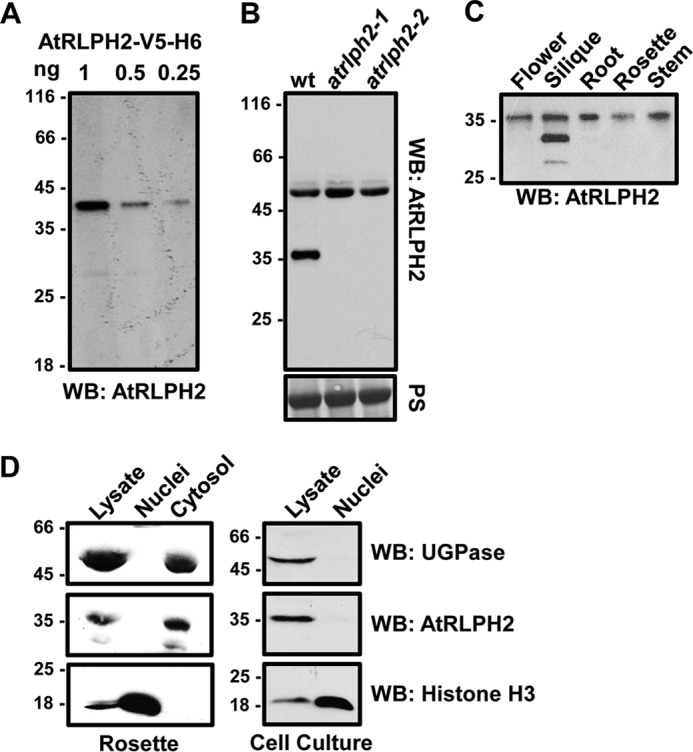
AtRLPH2 is a widely expressed cytosolic enzyme. A, affinity-purified anti-AtRLPH2 IgG (2 μg/ml) detected down to 0.25 ng of purified AtRLPH2-V5-H6 by Western blotting (WB). B, AtRLPH2 antibody (2 μg/ml) was used to probe either a WT or two separate AtRLPH2 T-DNA insertion line crude extracts (atrlph2-1, atrlph2-2). Prior to Western blotting, the membrane was Ponceau S (PS)-stained for equal loading (lower panel). AtRLPH2 is detected as an immunoreactive band at 36 kDa. C, extracts from various A. thaliana plant tissues probed with anti-AtRLPH2 IgG demonstrate broad tissue expression of AtRLPH2. D, crude lysate, isolated nuclei, and cytosolic fractions from A. thaliana rosettes (left panels) and cell culture (right panels) were Western blotted (WB) for AtRLPH2. Known cytosolic marker UDP-glucose pyrophosphorylase (UGPase) and nuclear marker histone H3 were used to verify the subcellular localization of AtRLPH2 and purity of each fraction. All mass markers are shown in kDa.
