FIGURE 1.
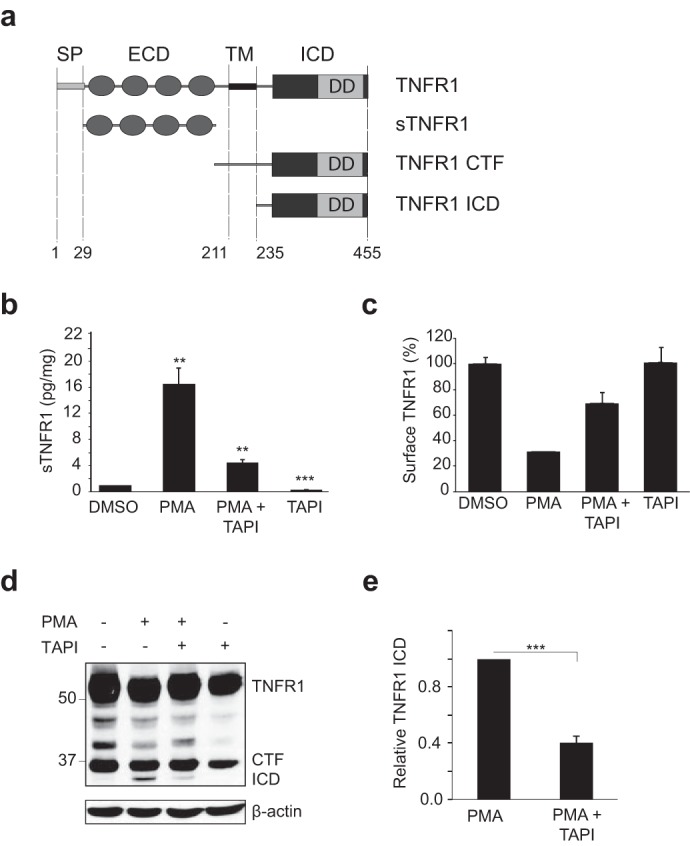
Ectodomain shedding is a prerequisite for γ-secretase cleavage of TNFR1. a, schematic representation of the domains of human TNFR1. Human TNFR1 (accession number AAA61201) is a 455-amino acid protein with a signal peptide (SP) at position 1–29; an extracellular domain (ECD) at position 29–211, containing four cysteine repeat domains; a 23-amino acid transmembrane domain (TM); and a 221-amino acid ICD at positions 235–455, containing the pro-apoptotic death domain (DD). Proteolytic cleavage by TACE/ADAM17 results in the generation of a soluble TNFR1 extracellular domain (sTNFR1) and membrane-anchored TNFR1 CTF. b, ELISA of sTNFR1 in conditioned medium from HEK293T cells transiently expressing TNFR1, left untreated or pretreated with TAPI-1 (50 μm for 2 h) and/or treated with PMA (200 ng/ml for 2 h). Data are expressed as a pictogram of sTNFR1/mg of total protein ± S.E. (error bars) versus TNFR1 control (n = 3). **, p < 0.01 (two-way ANOVA). c, FACS analysis of TNFR1 expression in HEK293T cells left untreated or pretreated with TAPI-1 (50 μm for 2 h) and/or treated with PMA (200 ng/ml for 2 h). Cells were stained with anti-TNFR1 and Alexa Fluor anti-mouse 488 antibodies. d, immunoassay of TNFR1 in corresponding whole cell lysates from HEK293T cells transiently expressing TNFR1 and left untreated or treated as in b and c. e, densitometry analysis of TNFR1 ICD normalized to total β-actin for all experimental conditions. The amount of TNFR1 ICD is expressed as TNFR1 ICD immunoreactivity ± S.E. (n = 3). ***, p < 0.001 (unpaired t test). Western blotting data are from one experiment representative of three independent experiments.
