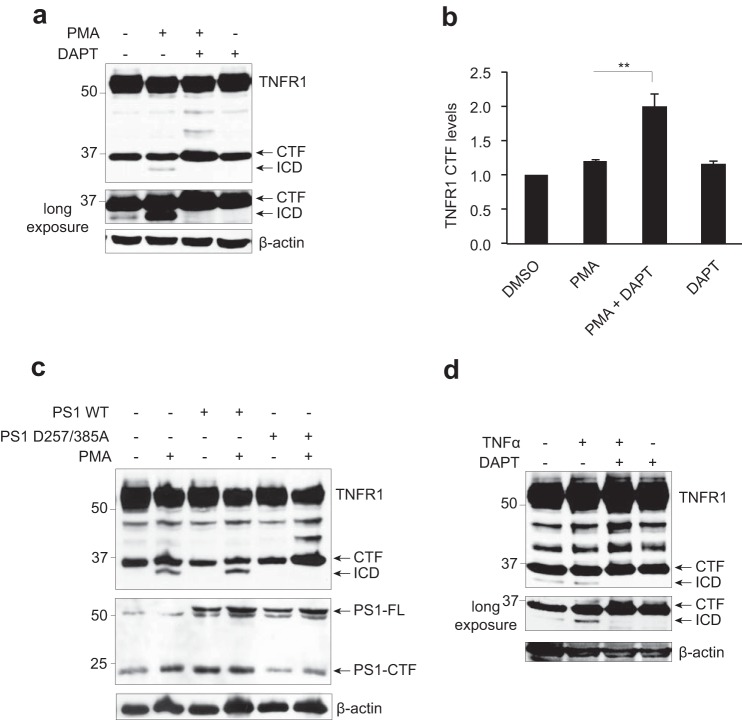
FIGURE 2.
TNFR1 is a substrate for γ-secretase-dependent regulated intramembrane proteolysis. a, immunoassay of TNFR1 in whole cell lysates from HEK293T cells transiently transfected with TNFR1 left untreated or pretreated with the γ-secretase inhibitor DAPT (10 μm for 8 h) and/or treated with PMA (200 ng/ml for 2 h), followed by Western blotting analysis with an anti-TNFR1 C terminus-specific antibody. b, densitometry analysis of TNFR1 CTF normalized to total β-actin for all experimental conditions (n = 3). **, p < 0.01 (two-way ANOVA). Error bars, S.E. c, immunoassay of cell lysates from HEK293T cells transiently expressing TNFR1 and co-expressing wild type PS1 or dominant negative PS1 (PS1D257A/D385A) mutant, left untreated or treated with PMA (200 ng/ml for 2 h) as indicated, followed by Western blotting analysis with an anti-TNFR1 (C terminus-specific), anti-PS1 (C terminus-specific), and anti-β-actin antibodies. d, immunoassay of whole cell lysates from HEK293T cells transiently expressing TNFR1, pretreated with the γ-secretase inhibitor DAPT (10 μm for 8 h), alone or in combination with TNFα (30 ng/ml for 2 h) as indicated, followed by Western blotting analysis with an anti-TNFR1 C terminus-specific antibody. Western blotting data are from one experiment representative of three independent experiments.