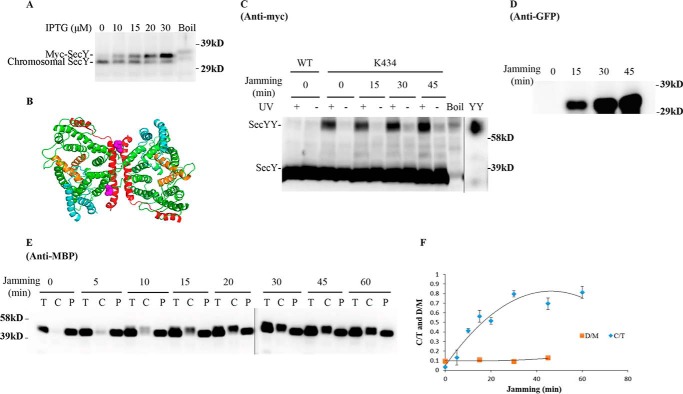
FIGURE 5.
Analysis of the secY Lys434 mutant during OmpA-GFP-induced translocon jamming by in vivo photo-cross-linking. A, titration of SecY expression with IPTG. The secY Lys434 mutant was grown in the presence of 1 mm pBpA until A600 reached 0.15, when IPTG was added to the indicated final concentration and growth continued for another 60 min. Cells were harvested and membranes were isolated and analyzed by Western blotting using anti-SecY peptide antiserum. B, view of T. maritima SecYEG docked in the back-to-back conformation with the same color scheme as described in the legend to Fig. 1. Residue Lys434 is shown as magenta spheres. C–F, the secY Lys434 mutant was grown in the presence of 1 mm pBpA, 30 μm IPTG, and 0.2% maltose until A600 reached 0.15, when the OmpA-GFP chimera was induced by adding arabinose to a final concentration of 0.2%. Cells were harvested at the indicated time points post jamming, and exposed to UV irradiation as indicated. A wild-type (WT) strain was used in parallel as a control. C, Western blot of cell membranes probed with c-Myc antibody. D, Western blot of cell membranes probed with GFP antibody. E, Western blot of cells divided into cytoplasm/membrane (C) and periplasmic (P) fractions, compared with total (T) cell input, probed with MBP antibody. F, quantification of the SecY dimer to monomer ratio (D/M) or cytoplasm/membrane MBP to total MBP ratio (C/T) during jamming. The average results from three experiments are plotted with standard error measurements. The error bars for the D/M points are too small to be seen on this scale.