FIGURE 7.
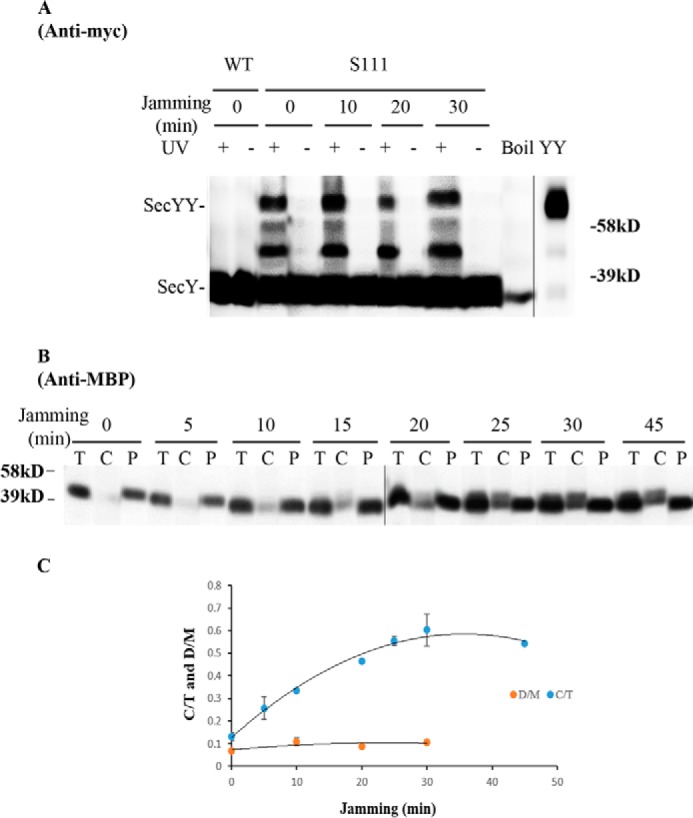
Analysis of the secY Ser111 mutant during MalE-LacZ-induced translocon jamming by in vivo photo-cross-linking. A–C, MM18.7 containing the pSup-pBpARS-6TRN and pCDFT7secYmycEG plasmids with the secY Ser111 amber mutation was grown in the presence of 1 mm pBpA and 30 μm IPTG until A600 reached 0.15, when the MalE-LacZ chimera was induced by adding maltose to a final concentration of 0.2%. Cells were harvested at the indicated time points post jamming, and exposed to UV irradiation as indicated. A wild-type (WT) strain was used in parallel as a control. A, Western blot of cell membranes probed with c-Myc antibody. B, Western blot of cells divided into cytoplasm/membrane (C) and periplasmic (P) fractions, compared with total (T) cell input, probed with MBP antibody. C, quantification of SecY dimer to monomer ratio (D/M) or cytoplasmic/membrane MBP to total MBP ratio (C/T) during jamming. The average results from three experiments are plotted with standard error measurements. The error bars for the D/M points are too small to be seen on this scale.
