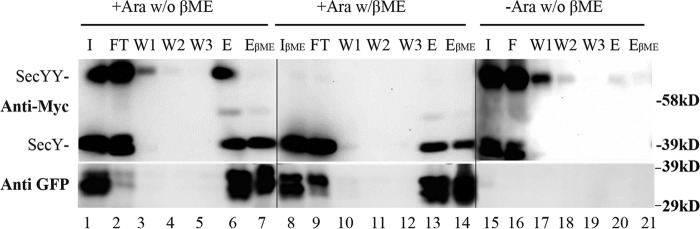
FIGURE 9.
Analysis of OmpA-GFP-jammed secY complex by co-immunoprecipitation. The secY Q212C mutant was grown in the presence of 30 μm IPTG to an A600 of 0.15, when arabinose was added as indicated to a final concentration of 0.2%, and growth was continued for an additional 45 min. Cells were harvested and treated with CuPhe3 to cross-link SecY prior to purification. Membranes were isolated and solubilized at 4 °C for 3 h in TSGM buffer containing 2% (w/v) DDM without (w/o βME) or with (w/βME) 5% β-mercaptoethanol as indicated, and insoluble material was removed by high-speed sedimentation. Solubilized membrane protein was mixed with anti-GFP beads at 4 °C for 2 h, when beads were sedimented, washed, and the sample eluted as described by the supplier. Western blot of the total input (I) of solubilized membrane protein, the flow-through (FT) fraction, three consecutive washes (W1, W2, and W3), and specifically eluted protein without (E) or with (EβME) treatment with 5% β-mercaptoethanol are shown. Western blots were probed with c-Myc or GFP antibody as indicated. Given the rapidly folding GFP domain, OmpA-GFP can run as a doublet (30).