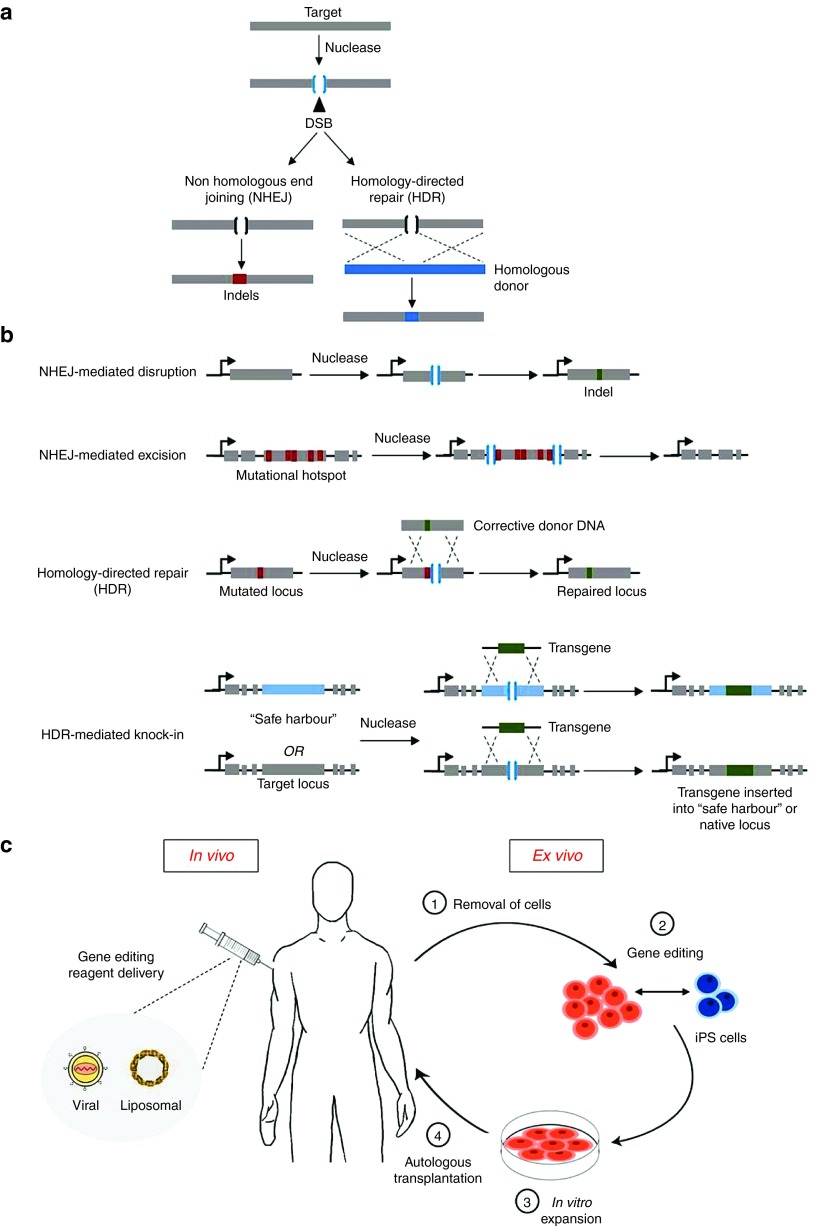
Figure 1.
Overview of therapeutic gene editing. (a) DNA repair pathways for the resolution of a double stranded break (DSB). A nuclease is targeted toward a defined genomic locus, introducing a DSB. This may undergo one of two major repair pathways known as nonhomologous end joining (NHEJ) or homology-directed repair (HDR), depending on cell cycle stage and availability of a DNA donor template. NHEJ is an error-prone mechanism, which causes small insertions or deletions (Indels) upon ligating the ends of the DNA break. HDR is a precise mechanism which repairs the break by using a homologous donor template. (b) Functional gene-editing strategies using DNA repair pathways. 1) NHEJ can be used to disrupt genomic sequences as a consequence of Indels. This can cause frameshift mutations leading to an early stop codon (or restoration of the reading frame by splice site disruption). 2) NHEJ can mediate targeted deletions. This requires generation of DSBs on both sides of the target genomic sequence, which then deletes the intervening sequence while NHEJ re-joins the DNA ends. 3) HDR can be used to correct a specific mutation by introducing a nuclease-mediated DSB (in proximity to the target site) in the presence of a homologous donor DNA containing corrective sequence. Upon recombination, the repair template corrects the mutated locus. 4) Likewise, by supplying exogenous DNA on the donor template flanked between regions of homology, HDR can be used to mediate targeted gene insertion or knock-in. (c) Schematic diagram comparing ex vivo and in vivo approaches. In vivo approaches involve direct transfer (denoted by the syringe) of genome-editing reagents such as programmable nucleases and donor templates to the human body. In this instance, two prominent gene transfer agents, viral vectors, and liposomes are shown. Ex vivo is centered on correction of the genetic defect outside of the body. This is a staged-approach whereby: 1) Patient cells are obtained. 2) Gene editing is performed in vitro. This involves delivery of nucleases on their own or concomitantly with repair template. The patient cells can be programmed into induced pluripotent stem cells (iPSCs) before or after gene editing. Once corrected, iPSCs may be differentiated into cell types of interest. 3) The genetically corrected cells are characterized and expanded. 4) The corrected cells are then re-grafted back into the patient through autologous transplantation.