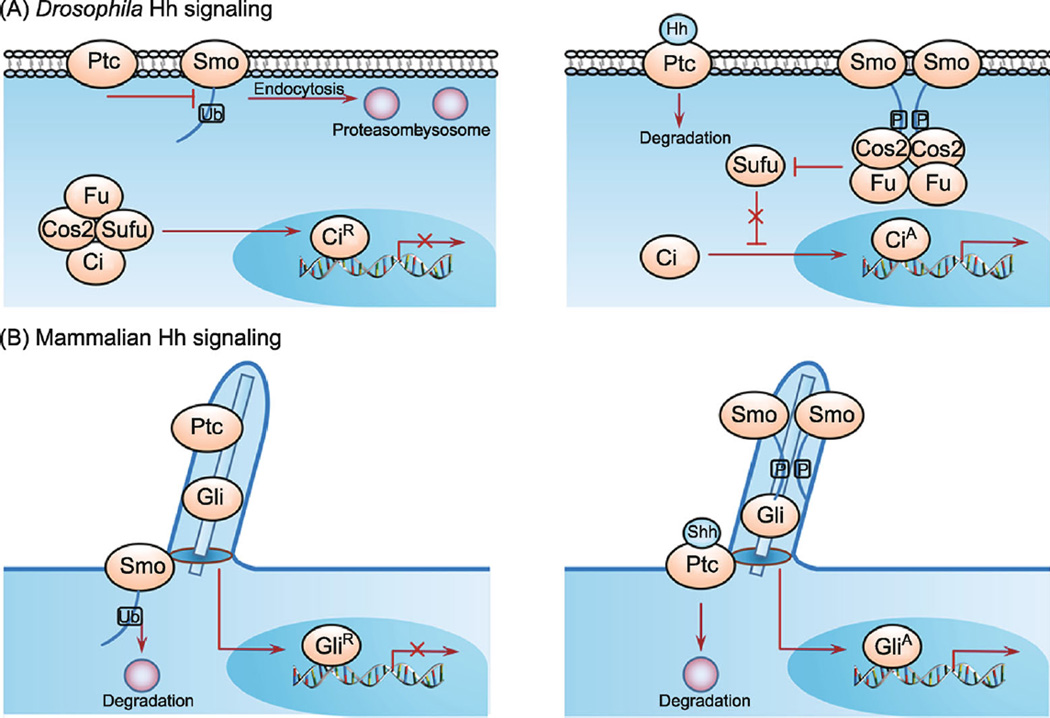
Figure 1.
The regulation of Smo phosphorylation and ubiquitination in Drosophila and mammalian Hh signaling pathways. (A) Schematic drawings of Drosophila Hh signaling. In the absence of Hh (left panel), Ptc inhibits Smo. Smo is ubiquitinated at multiple residues among its C-tail, resulting in endocytosis and degradation by both lysosome- and proteasome-dependent pathways. Full-length Ci (CiF) undergoes proteolytic processing to generate a truncated repressor form (CiR), which blocks the expression of Hh target genes. In the presence of Hh (right panel), binding of Hh to Ptc releases its inhibition on Smo and triggers phosphorylation of Smo by multiple kinases (PKA, CK1, CK2, Gprk2 and aPKC), leading to Smo cell surface accumulation, dimerization and activation, thus the conversion of CiF into the activator form CiA. (B) Schematic drawings of mammalian Hh signaling in the cilium. In the absence of Shh (left panel), Smo is excluded from the primary cilium, where Ptc resides to inhibit Hh signaling. In the presence of Shh (right panel), binding of Shh to Ptc leading its departure from primary cilium, where Smo is accumulated, phosphorylated, dimerized and activated. The active forms of Gli (GliA) induce Hh target gene expression.