Figure 6.
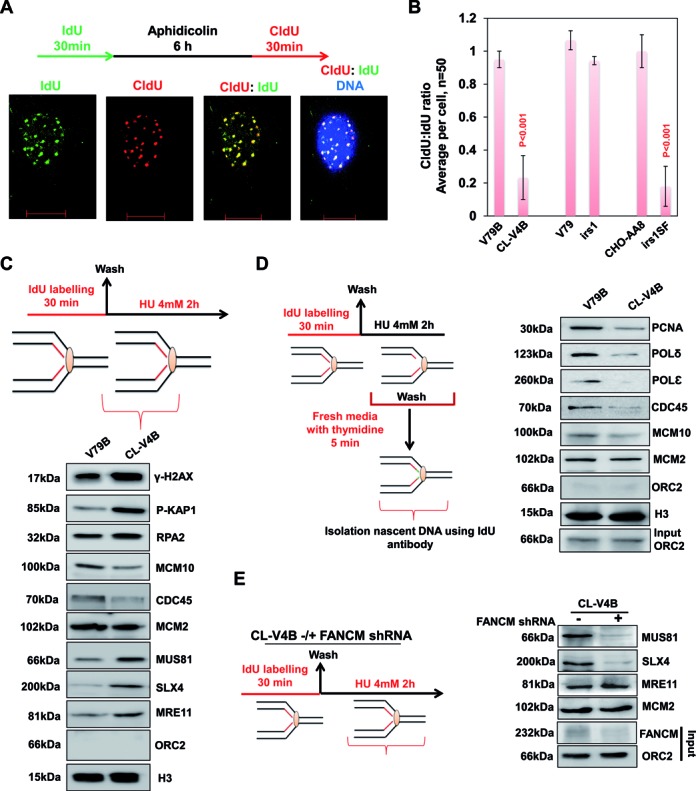
RAD51C deficiency leads to loss of replisome components from stalled forks and impairs restart. (A) Representative image for replication restart after aphidicolin treatment. (B) Quantification of IF data in indicated RAD51 paralog defective cells. Data are mean ± SD (n = 3). Quantification of replication restart after aphidicolin treatment in XRCC3 depleted irs1 and V-C8 cells (BRCA2−/−). P-values are obtained for paralog or BRCA2 deficient cells compared to respective parental cells. (C) Experimental design for nascent strand pull-down of HU-mediated stalled replication forks. wt V79B or RAD51C−/- CL-V4B cells were labeled with IdU for 30 min, followed by release into 4 mM HU-containing medium for 2 h. Cells were cross-linked, and the chromatin fraction was isolated and subjected to IP using anti-IdU antibody. Fractions were probed for indicated proteins with Histone H3 as loading control. (D) Experimental design for monitoring fork recoupling after recovery from HU. HeLa cells were labeled with IdU for 30 min, followed by 4 mM HU treatment for 2 h. Later cells were released into thymidine-containing medium for 5 min, cross-linked, and the chromatin fraction was isolated (input) and subjected to IP using anti-IdU antibody. Fractions were probed for indicated proteins with ORC2 as control (input and negative control). (E) FANCM deficiency rescues excessive processing of stalled forks in RAD51 paralog deficient cells. RAD51C deficient CL-V4B cells treated with FANCM shRNA were labeled with IdU, followed by 4 mM HU treatment for 2 h. Cells were cross-linked, and the chromatin fraction was isolated (input) and subjected to IP using anti-IdU antibody. Fractions were probed for indicated proteins.
