Table 2. Data collection and refinement statistics.
| X-ray diffraction data | |
|---|---|
| Wavelength (Å) | 0.9725 |
| Space group | C2221 |
| Cell dimensions: a, b, c (Å) | 200.2, 200.2, 83.6 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Resolution (Å) | 47.2–3.8 (4.25–3.80) |
| Rmergea | 0.279 (0.58) |
| Rpimb | 0.107 (0.22) |
| I/σI | 5.2 (3.2) |
| Completeness (%) | 99.0 (99.2) |
| Redundancy | 7.5 (7.8) |
| Total measured reflections | 125 845 (36 404) |
| Unique reflections | 16 786 (4 686) |
| Wilson B-factor (Å2) | 50.2 |
| Refinement | |
| Resolution (Å) | 141.6–3.8 |
| Rcryst/Rfreec | 0.22/0.27 |
| Protein atoms | 7 336 |
| B factor (Å2) | 80.7 |
| RMSD bond length (Å) | 0.012 |
| RMSD bond angles (°) | 1.384 |
| Ramachandran analysis: favored/allowed (%) | 97.6/2.4 |
Values in parentheses are for the highest resolution shell.
a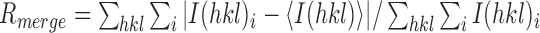 ,
,
b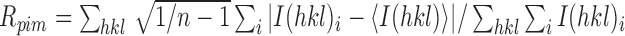 , where I(hkl) is the intensity of reflection hkl and 〈I(hkl)〉 is the average intensity over all equivalent reflections.
, where I(hkl) is the intensity of reflection hkl and 〈I(hkl)〉 is the average intensity over all equivalent reflections.
c . Rfree was calculated for a test set of reflections (5%) omitted from the refinement.
. Rfree was calculated for a test set of reflections (5%) omitted from the refinement.
