Figure 3.
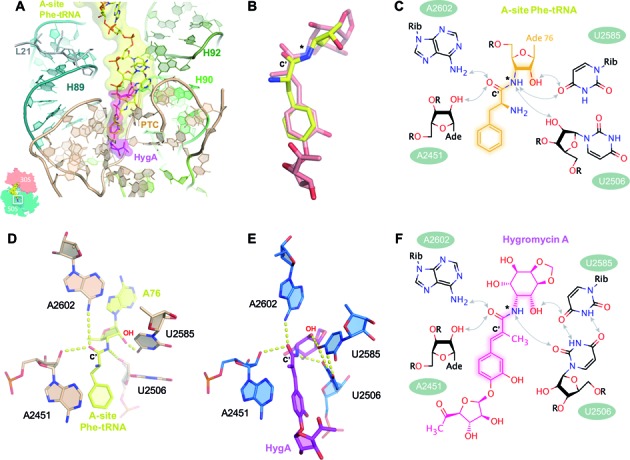
The binding site of HygA on the ribosome overlaps with that of the aminoacyl-A76 ribosyl moiety of the A-site tRNA. (A) The 50S–HygA HygA–5 complex was aligned to a 70S ribosome with an accommodated A-site (PDB accession code: 4QCP; (25)) using 23S rRNA residues 2063–2092, 2227–2258, 2435–2457 and 2494–2608 showing that the aminoacyl-A76 ribosyl moiety at the acceptor end of the A-site tRNA (yellow) overlaps with the binding site of HygA (magenta). In (B) only HygA and the aminoacyl-A76 ribosyl moiety from the aligned structures (panel A) are shown to highlight their similarity. When aligned using PTC residues (as shown in A) the 15 atoms spanning from the 3-hydroxyl to C7’ position of HygA (Supplementary Figure S1) and their counterparts in the A-site tRNA (indicated by highlights in the chemical structures shown in panels C and F) have a root mean square deviation (RMSD) of 1.57 Å. When these 15 atoms are directly aligned on themselves they have a RMSD of 0.86 Å. Both the aminoacyl moiety of the A-site tRNA (C and D) and HygA (E and F) appear to be coordinated by a similar hydrogen bond network that includes the 23S rRNA residues A2451 (via 2′-OH of the ribose), U2506 (2′-OH of the ribose in the acylated tRNA and O2 of the base in the 50S–HygA structure) and A2602 (via the N6 amino group) while U2585 in the 50S–HygA complex is kept at a position similar to that seen in PDB accession code: 4QCP (25) by forming a symmetric 4-carbonyl-N3 U-U pair with residue U2506. The carbonyl carbon present in both ligands is labeled as C’. Note that in a native aminoacyl-tRNA an ester oxygen rather than an amide nitrogen links the aminoacyl moiety to the tRNA (this position is indicated by an asterisk in panels B, C and F) that can serve as a proton acceptor only. However, an analogous hydrogen bond between the ester oxygen and U2506 can be formed if the uridine moiety is in the enol form having a hydroxyl group at position 2 acting as proton donor toward the electron pair of the aminoacyl ester oxygen.
