Figure 1.
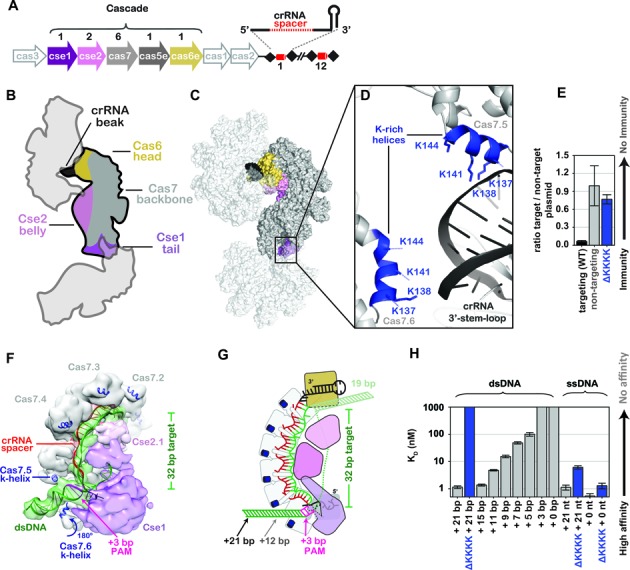
A lysine-rich vise is critical for binding double-stranded DNA (dsDNA). (A) The Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-mediated adaptive immune system in Escherichia coli (Type I-E) consists of eight cas genes (arrows) and a CRISPR locus (black diamonds and red cylinders). Five of the cas genes (colored arrows) encode proteins that assemble with a 61-nt crRNA to from the seahorse-shaped complex composed of an unequal number of five different Cas proteins. The stoichiometry of Cas proteins in Cascade is indicated above the arrows. (B) Cartoon schematic of how three symmetry-related Cascade complexes pack together in the crystal in a head-to-tail and head-to-belly arrangement. Subunits are colored according to the scheme used in panel A. (C) Surface rendition of symmetry-related Cascade complexes. (D) The interface between the 3′ stem-loop of one Cascade assembly with lysine-rich (K-rich) helices of another. Positively charged K-rich helices are indicated in blue. (E) Plasmid curing results. The ΔKKKK mutation results in immunodeficiency. (F) Model of Cascade bound to dsDNA docked into the cryo-EM density EMDB 5929. The K-helices of Cas7.5 and Cas7.6 are indicated. (G) Schematic of Cascade-binding dsDNA. The protospacer adjacent motif and number of base pairs required to reach through the K-helices (+12) are indicated. (H) Equilibrium dissociation constants of Cascade and ΔKKKK Cascade with various dsDNA and ssDNA substrates containing 3′-ends with variable lengths.
