Figure 3.
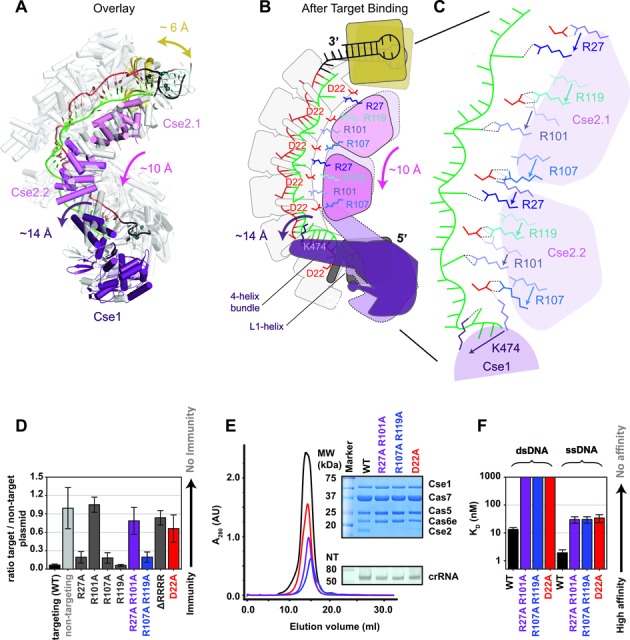
Conserved arginines on the Cse2 subunits participate in a relay that stabilizes target binding induced conformational changes. (A) Overlay of Cascade crystal structures before (gray) and after (colored) target binding. (B-C) Schematic of the conformational change induced by target binding. The two Cse2 subunits move ∼10-Å down the backbone of Cascade. Salt bridges between the belly (Cse2 R27 and R101) and the backbone (Cas7 D22) are broken. R27 and R101 are repositioned to stabilize the flipped-out bases on the DNA target strand and compensatory salt bridges are formed by Cse2 R107 and R119 with D22 residues on Cas7 subunits further down the backbone. (D) Plasmid-curing assays reveal that R101A mutations result in strong immune system defects, while individual mutation of the other Cse2 arginines shows little or no measurable defect. The D22A mutation also results in immunodeficiency. (E) Elution profile of the WT Cascade and different mutants. The insert shows a Commassie blue-stained SDS-PAGE gel (top) and a denaturing polyacrylamide gel of phenol extracted crRNA isolated for the Cascade complexes (bottom). The Cse2 R27A/R101A, Cse2 R107A/R119A and Cas7 D22A mutants lack Cse2. (F) Equilibrium dissociation constants of Cascade and Cascade mutants for a 72-bp dsDNA target and a 72-nt ssDNA target containing a 5′-CAT-3′ PAM.
