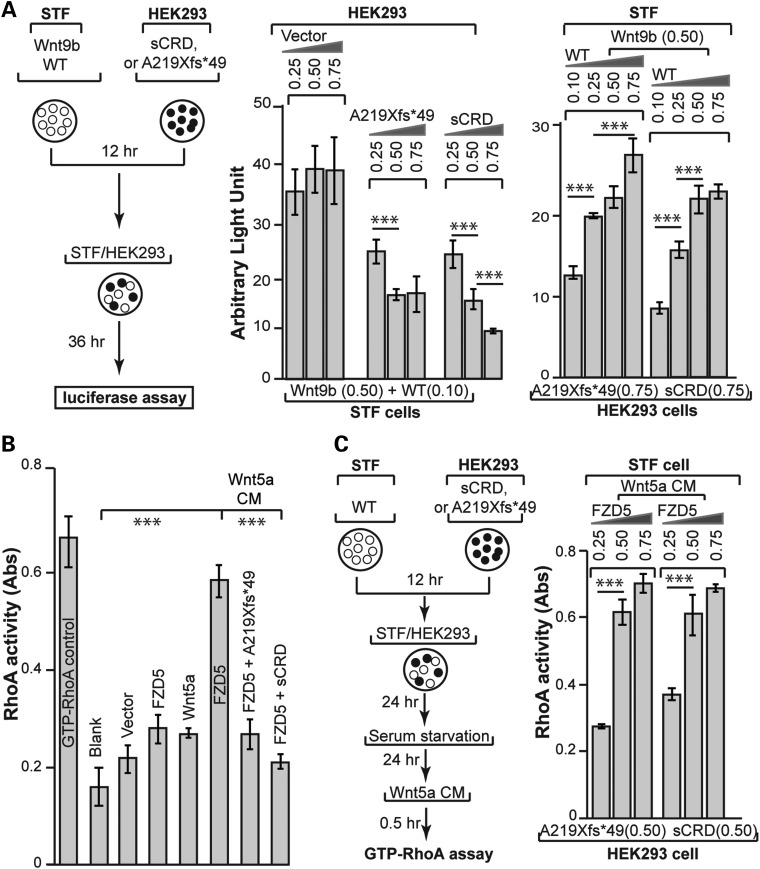
Figure 4.
Non-cell-autonomous dominant-negative effect of FZD5 A219Xfs*49 mutant on Wnt signaling. (A) Wnt9b–FZD5 signaling. All experiments were done in triplicates of at least three independent transfections. Left panel: illustration of the experimental scheme. A fixed amount of Wnt9b and FZD5 was co-transfected with pCAG-Renilla luciferase plasmids (RL, used for internal expression control) into STF cells. Different amounts of FZD5 A219Xfs*49 and sCRD plasmids were transfected into HEK293 cells. After 12 h, both STF and HEK293 cells were collected by trypsin-Ethylenediaminetetraacetic acid (EDTA), mixed at 1:1 ratio and seeded into a new plate for another 36 h. Cell extracts were then prepared for Firefly luciferase and Renilla luciferase assay. Middle panel: inhibition of Wnt9b/FZD5 activity by either A219Xfs*49 or sCRD in a dose-dependent manner. Firefly luciferase activities were normalized against Renilla luciferase, and statistics was performed using the Microsoft Excel software. Right panel: the inhibition of FZD5-mediated Wnt signaling by A219Xfs*49 or sCRD was reversed by augmenting FZD5 expression. (B) Wnt5a–FZD5 signaling. RhoA G-lisa assay showed that Wnt5a/FZD5-stimulated accumulation of GTP-RhoA was abolished by A219Xfs*49 mutant or sCRD protein (compare the right three bars). Samples preparation was as described in Figure 3D, G-lisa assay followed instructions of RhoA G-lisa kit (Cytoskeleton, Inc.). Absorbance of horseradish peroxidase colorimetric reaction was measured by SpectraMax M. The data were quantified by the Microsoft Excel. (C) Inhibition of RhoA activation by A219Xfs*49 or sCRD protein was reverted by increased FZD5 expression. Left panel: similar experimental scheme in (A) was used for testing non-cell-autonomous effects of A219Xfs*49 on Wnt5a/FZD5 induced RhoA activation. Right panel: The inhibition of RhoA activation (G-lisa assay) was reverted by increased FZD5 expression. ***P < 0.001, Student's t-test.