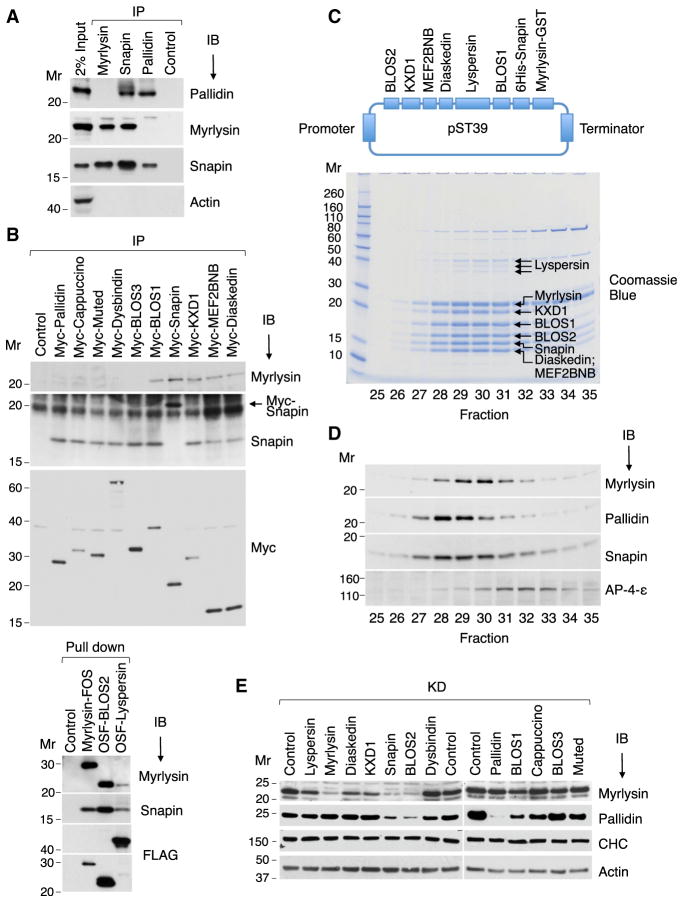
Figure 2. Biochemical Characterization of BORC.
(A) HeLa cell extracts were subjected to immuno-precipitation (IP) followed by immunoblotting (IB) with the indicated antibodies.
(B) HeLa cells stably expressing Myc-, FOS-, or OSF-tagged subunits of BORC and BLOC-1 were extracted with lysis buffer and subjected to immunoprecipitation with antibody to the Myc epitope or pull-down with Strep-Tactin beads. Endogenous myrlysin and Snapin were then detected by immunoblotting. The ~20-kDa band in the Snapin IB of the Myc-Snapin IP lane (arrow) corresponds to Myc-Snapin.
(C) cDNAs encoding BORC subunits, including His6-Snapin and myrlysin-GST, were cloned into the E. coli expression plasmid pST39 (top). Purified BORC was cleaved with TEV protease to remove the tags and analyzed by gel filtration on Superose 6. Fractions 25–35 were resolved by SDS-PAGE, and proteins were visualized by Coomassie blue staining (bottom) and their identity confirmed by MS (Table S2).
(D) H4 cell extracts were analyzed by gel filtration under the same conditions as in (B). Endogenous myrlysin, pallidin, Snapin, and the adaptor protein 4 (AP-4) ε subunit (control) were detected by immunoblotting.
(E) siRNA-KD of BORC and BLOC-1 subunits was performed in HeLa cells, and myrlysin, pallidin, clathrin heavy chain (CHC), and actin (the latter two as controls) were detected by immunoblotting. The positions of molecular mass (Mr) markers (in kilodaltons) are indicated.