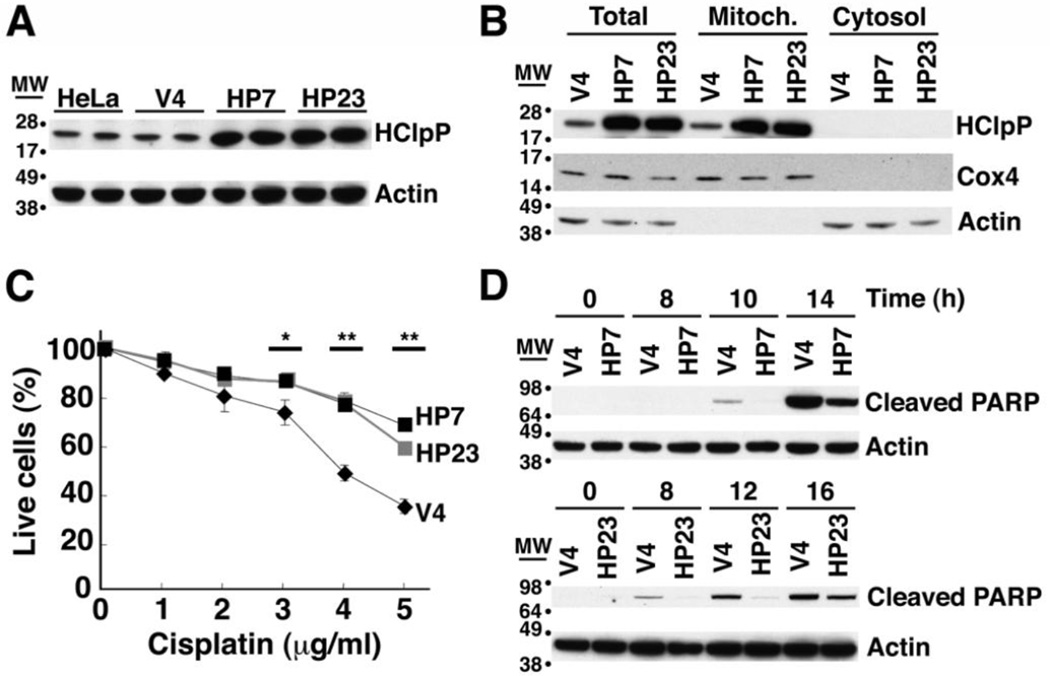
Fig. 1. Excess of HClpP protects HeLa cells from killing by cisplatin.
A. HClpP expression in stable transfectants. Proteins were extracted from stable transfectants of pTRE2-HClpP (HP7 and HP23) or the pTRE2 vector (V4) and separated by SDS-PAGE. HClpP was detected by western blotting with anti-HClpP antibodies. Actin was monitored as a loading control using an antibody against the C-terminal peptide common to α, β, and γ actins. B, Subcellular distribution of HClpP in stable transfectants. Mitochondrial and cytosolic fractions were prepared from HP7, HP23, and V4 cells. Protein aliquots (25 µg) from each fraction were separated by SDS-PAGE, and HClpP was measured by western blotting with antibodies to HClpP. Separation of mitochondria and cytosol was confirmed with antibodies to Cox4 as a mitochondrial marker or actin as a cytosolic marker. C, Sensitivity of transfectants to cisplatin. V4, HP7, and HP23 cells were treated with 0–5 µg/ml cisplatin (CP) for 16 h, and cell viability was measured by CCK-8 as described in "Materials and Methods". The viability of cells without cisplatin treatment was normalized to 100%. Results from a single experiment are shown. Error bars represent the standard error of measurements made in triplicate, and significant differences between overexpression and control cells determined by a two-tailed T-test are noted with asterisks. Similar results were obtained in at least three independent experiments. D, Delayed apoptotic response in HClpP-transfectants treated with cisplatin. Apoptotic cell death of cells treated with cisplatin was monitored by western blotting to detect the cleavage product of PARP, a marker for late stages of apoptosis. Aliquots (25 µg protein) from V4, HP7, and HP23 cells that had been treated with 5 µg/ml cisplatin were separated by SDS-PAGE and probed with an antibody specific for the proteolytically processed form of PARP. Actin served as the loading control.