Abstract
The proliferation of CRISPR/Cas9-based methods in Caenorhabditis elegans has enabled efficient genome editing and precise genomic tethering of Cas9 fusion proteins. Experimental designs using CRISPR/Cas9 are currently limited by the need for a protospacer adjacent motif (PAM) in the target with the sequence NGG. Here we report the characterization of two modified Cas9 proteins in C. elegans that recognize NGA and NGCG PAMs. We found that each variant could stimulate homologous recombination with a donor template at multiple loci and that PAM specificity was comparable to that of wild-type Cas9. To directly compare effectiveness, we used CRISPR/Cas9 genome editing to generate a set of assay strains with a common single-guide RNA (sgRNA) target sequence, but that differ in the juxtaposed PAM (NGG, NGA, or NGCG). In this controlled setting, we determined that the NGA PAM Cas9 variant can be as effective as wild-type Cas9. We similarly edited a genomic target to study the influence of the base following the NGA PAM. Using four strains with four NGAN PAMs differing only at the fourth position and adjacent to the same sgRNA target, we observed that efficient homologous replacement was attainable with any base in the fourth position, with an NGAG PAM being the most effective. In addition to demonstrating the utility of two Cas9 mutants in C. elegans and providing reagents that permit CRISPR/Cas9 experiments with fewer restrictions on potential targets, we established a means to benchmark the efficiency of different Cas9::PAM combinations that avoids variations owing to differences in the sgRNA sequence.
Keywords: alternate PAMs, C. elegans, CRISPR/Cas9, genome editing, VQR Cas9
CHARACTERIZATION of the type II CRISPR/Cas bacterial immunity system has provided a powerful and efficient means to tailor the genomes of a wide variety of organisms (Cong et al. 2013; DiCarlo et al. 2013; Dickinson et al. 2013; Friedland et al. 2013; Gratz et al. 2013; Li et al. 2013; Yang et al. 2014). The Cas9 endonuclease can be programmed by a readily modified single-guide RNA (sgRNA) to cleave a target DNA sequence (Jinek et al. 2012). In vivo, this activity can result in imperfect repair by nonhomologous end joining (NHEJ), often creating a null allele of a target gene. When a homologous donor template is provided, desired modifications can be precisely introduced through homologous recombination (HR). The affinity of Cas9 for targeted sequences also has been harnessed to directly influence gene expression. The use of catalytically inactive Cas9 fused to transcriptional activators, repressors, or histone-modifying enzymes has shown that the full potential of CRISPR/Cas9 techniques is still being realized (Gilbert et al. 2013; Mali et al. 2013; Hilton et al. 2015; Kearns et al. 2015; Long et al. 2015).
All these methods share the requirement of a protospacer adjacent motif (PAM) in the target (Jinek et al. 2012). The NGG PAM required by the widely used Cas9 from Streptococcus pyogenes is relatively abundant in randomly composed DNA but is less common in AT-rich genomes, imposing a significant constraint. Recent studies have explored the utility of Cas9s from other bacterial species with diverse PAM specificities, as well as the directed evolution of the S. pyogenes Cas9 coding sequence to select mutants that recognize alternate PAMs (Esvelt et al. 2013; Kleinstiver et al. 2015; Ran et al. 2015).
Several groups have developed effective protocols using CRISPR/Cas9 and additional tools for the introduction of specific mutations into the Caenorhabditis elegans genome (Dickinson et al. 2013, 2015; Arribere et al. 2014; Kim et al. 2014; Paix et al. 2014, 2015; Zhao et al. 2014; Ward 2015). One particularly flexible procedure involves injection of the gonad with a Cas9 transgene, a sgRNA expression vector, and an oligonucleotide repair template, resulting in gene conversion via HR with the donor template (Arribere et al. 2014; Paix et al. 2014; Zhao et al. 2014). Co-conversion with a dominant phenotypic marker mutation reduces the amount of screening necessary to isolate a desired mutant (Arribere et al. 2014; Kim et al. 2014; Ward 2015). In our application of this technique, we have observed that the recombination with the oligonucleotide donor is highly localized (Arribere et al. 2014). The portion of the donor incorporated into the genome often extends only 10 or fewer bases from the site of Cas9 cleavage. In addition, a scarcity of GG dinucleotides in many regions of the genome has confounded targeting certain types of functionally critical sequences, such as 3′ UTRs. As a result, we were motivated to make more of the C. elegans genome accessible to efficient editing with oligonucleotide templates by expressing Cas9s with a variety of PAM requirements. In choosing an approach to extend the targetable genome, it was important to consider the substantial challenges that have been encountered in expressing foreign coding sequences in the nematode germline (Kelly et al. 1997). Expression of a “new” coding sequence in the germline can take several trials, with success or failure occurring as a complex function of the corresponding DNA, RNA, and protein sequences (Fire 2005). This provides a distinct advantage to approaches for extending editing technology that rely on coding regions that derive from established vectors that are active in the germline. In this work, we describe a set of variants of an effective S. pyogenes Cas9 germline expression vector.
To extend the functional editing repertoire in C. elegans, we investigated two recently described Cas9 variants that appeared particularly promising based on their reported specificities (Kleinstiver et al. 2015). One mutant, termed the VQR variant, possesses the amino acid substitutions D1135V, R1335Q, and T1337R, which results in a Cas9 protein that recognizes an NGA PAM. The second mutant, referred to as the VRER variant, has the mutated residues D1135V, G1218R, R1335E, and T1337R, which enable recognition of an NGCG PAM (Figure 1A) . In the crystal structure of wild-type Cas9 in complex with a sgRNA and an NGG PAM, the R1335 residue, substituted in both the VQR and VRER variants, contacts the third PAM position (Anders et al. 2014). The T1337R substitution present in both variants is also located in this conserved PAM-interacting domain. The mutant proteins recognize the same sgRNA scaffold (secondary structure) as wild-type S. pyogenes Cas9, a substantial advantage in working with these variants.
Figure 1.
Construction of C. elegans transgenes expressing Cas9 mutants with alternative PAM preferences. (A) Partial amino acid sequences of the S. pyogenes Cas9 PAM-interacting domain and mutants that have been evolved (Kleinstiver et al. 2015) to recognize an NGA PAM or an NGCG PAM. Altered residues are numbered and highlighted in red. (B) A representation of the Cas9 transgene modified to express each variant (Dickinson et al. 2013). The transgene was driven ubiquitously by the eft-3 promoter and contains three synthetic introns and the 3′ UTR from tbb-2. Asterisks denote the locations of the mutations introduced to generate the variants.
Materials and Methods
Strain construction and maintenance
The VC2010 N2 strain (Thompson et al. 2013) was used as an initial starting strain for all experiments described in this paper. The strain was used directly in experiments to test the VQR and VRER Cas9 variant transgenes in dpy-10 and unc-58 and to assess their PAM specificity via co-injection with the wild-type Cas9 transgene. When directly comparing the VQR transgene with wild-type Cas9 and the VRER variant using the same sgRNA vector, the VQR transgene was injected into VC2010 animals, whereas the other two were injected into mutant derivatives of VC2010 with modified PAMs (NGG or NGCG) at the dpy-10 locus. The VC2010 variants needed for the wild-type and VRER assays were generated using a two-step oligonucleotide-templated CRISPR/Cas9 editing approach (Arribere et al. 2014). VC2010 animals were first altered to generate dpy-10(cn64) homozygotes, which have a Dpy Rol phenotype that is easily distinguished from Rol heterozygotes. Subsequent use of a sgRNA vector targeting the dpy-10(cn64) sequence facilitated recombination of oligonucleotides carrying silent mutations to alter the endogenous PAM and to revert the dpy-10(cn64) mutation, yielding Rol animals. From this group of Rol progeny, wild-type progeny were isolated and validated as the desired mutants by PCR and sequencing. All strains used to test each of the four NGAN PAMs with the VQR Cas9 variant were constructed in the same fashion. All strains were grown on nematode growth medium (NGM) plates seeded with Escherichia coli strain OP50 (Brenner 1974). All animals were grown at 16° prior to injection and at 23° after injection. Supporting Information, Table S1 lists all strains created and used in this study.
Plasmid construction
Plasmids have been deposited with Addgene with the following accession numbers: VQR Cas9 (pRB1080), 71309; VQR dpy-10 sgRNA plasmid (pRB1081), 71479; VRER Cas9 (pRB1083), 71480; and VRER dpy-10 sgRNA plasmid (pRB1084), 71516. All other plasmids used in this study are available on request.
The VQR and VRER Cas9 transgenes (pRB1080 and pRB1083) were constructed by Gibson cloning (Gibson et al. 2009) of gBlocks Gene Fragments (Integrated DNA Technologies) into the vector pDD162 (Peft-3::Cas9::tbb-2 3′ UTR). Each gBlock contained a portion of the Cas9 coding sequence from pDD162 with codon substitutions to express the mutated proteins. These codons were optimized for worm expression using the C. elegans codon adaptor (Redemann et al. 2011). The sgRNA expression vectors pRB1081 (VQR, dpy-10), pRB1082 (VQR, unc-58), pRB1084 (VRER, dpy-10), pRB1085 (VRER, unc-58), and pRB1086 (wild-type Cas9, dpy-10(cn64)) were built by cloning oligonucleotides into the vector pRB1017 (Arribere et al. 2014). The plasmids were verified by sequencing, and their concentrations were determined by the Qubit dsDNA BR Assay Kit (Invitrogen). Table S2 lists all vectors, oligonucleotides, and gBlocks used in this study.
Microinjection
In all injections, Cas9 plasmid was present at 50 ng/μl such that when two different Cas9 transgenes were in the same injection mix, each was present at 25 ng/μl. Each of the sgRNA plasmids was present at 25 ng/μl, and each donor oligonucleotide was present at 500 nM in all injections regardless of the number in the mix. Young-adult animals were injected in both distal gonad arms in most cases. Injected animals were rehydrated in recovery buffer (1 mg/ml salmon sperm DNA, 4% glucose, 2.4 mM KCl, 66 mM NaCl, 3 mM MgCl2, 3 mM CaCl2, and 3 mM HEPES, pH 7.2) prior to being placed on an NGM plate seeded with OP50.
Screening
The F1 generation was screened for Rol, Dpy, and Unc phenotypes 3–4 days after injection. The progeny of animals injected with a mixture targeting dpy-10 for conversion consistently displayed a range of phenotypes besides the left-handed rolling exhibited by dpy-10(cn64) mutants and the Dpy phenotype of dpy-10 null homozygotes. In practice, it can be difficult to distinguish between slightly Dpy animals, which can be dpy-10(cn64) heterozygote mosaics that hardly roll; heterozygotes for a dpy-10 null mutation; and animals that have no heritable dpy-10 mutation. We attribute the indistinct range of phenotypes we observed to mosaicism and the obfuscating effect of Cas9 activity in somatic tissue. In our experience observing the progeny of these individuals, we have discerned that most carry heritable dpy-10 null mutations but that a significant fraction possesses the dpy-10(cn64) mutation resulting from HR with the donor template, while others have no heritable phenotype. For our purposes, we scored any ambiguous phenotypes with partial or complete dumpiness and any degree of body shape dysfunction as Dpy Rol. When Dpy or Rol animals were also Unc, they were scored for consistency as a single class (Dpy Unc or Rol Unc).
Calculation of newly targetable sequences
The C. elegans annotated RefSeq genes were downloaded using the University of California–Santa Cruz (UCSC) Table Browser and the WS220/ce10 genome assembly. Each was downloaded with sufficient flanking sequence to determine the sgRNA sequence for Cas9 cleavage sites near the ends of each gene, but only bases within the coding sequence were checked for proximity to a potential Cas9 cleavage site. Intron sequences were included for sgRNA sequence determination but were not considered for the proximity calculation.
C. elegans 3′ UTR sequences were obtained from the coordinates published in Jan et al. (2011). The sequences were downloaded using the UCSC Table Browser and the WS190/ce6 genome assembly and included sufficient flanking sequence to determine the sgRNA sequence for potential Cas9 cleavage sites near the ends of each 3′ UTR, but only bases within the 3′ UTR sequence were considered for the proximity calculation. The analysis was performed using custom Python Scripts.
Results and Discussion
Two transgenes expressing Cas9 variants with alternate PAM specificities promote oligonucleotide-templated HR at multiple target loci
We introduced the sequence changes leading to the VQR and VRER Cas9 mutants into a construct that drives ubiquitous Cas9 expression in C. elegans with the promoter from eft-3 (Figure 1B) (Dickinson et al. 2013). In the characterization of the VQR and VRER Cas9 variants by Kleinstiver et al. (2015), the assays employed to demonstrate function measured the disruption of genes by indel mutations resulting from NHEJ. Because homologous recombination using a defined template (HR) has been a key component in much of CRISPR/Cas9-mediated genome editing in C. elegans, we used a protocol that would detect both types of events. We co-injected each Cas9 variant plasmid with a sgRNA expression vector and an oligonucleotide to introduce mutations causing the easily scored Rol and Dpy phenotypes characteristic of dpy-10(cn64) and dpy-10 loss of function, respectively (Figure 2, A and B) (Levy et al. 1993) . We observed that each transgene was indeed capable of inducing both HR and NHEJ events at dpy-10. The editing process with these Cas9 variants in C. elegans was efficient: most injected worms produced animals with modified targets among their progeny. These events were heritable, as expected for germline modification of the locus. The confirmation of germline activity was important because somatic NHEJ events at dpy-10 often can produce a nonheritable Dpy phenotype. In these experiments, the efficiency of the VQR variant at dpy-10 appeared comparable to that of wild-type Cas9 acting at a similar site. While the VRER variant was able to generate both HR and NHEJ events, it appeared to be somewhat less active than either wild-type Cas9 or the VQR variant in the dpy-10 assay. Additional support for the function of the VQR and VRER variants was provided by HR assays with the unc-58 gene (Figure 2, A and C) (Arribere et al. 2014). Although the efficiency at unc-58 was low for both variants compared to the wild-type Cas9 transgene, the appearance of worms with the unique shaking phenotype characteristic of the intended point mutant (unc-58(e665)) provided a confirmation of effective HR.
Figure 2.
Oligonucleotide-templated HR in multiple C. elegans genes using Cas9 variants requiring NGA and NGCG PAMs. (A) Schematic of the technique used to induce readily identifiable homologous replacement in a genomic target. A Cas9 germline transgene, a vector driving sgRNA expression with a C. elegans U6 promoter, and a homologous oligonucleotide donor template bearing a dominant phenotypic marker mutation were injected into the gonads of young-adult animals. Recombination at the endogenous locus was demonstrated by the observation of Rol or Unc phenotypes in the F1 progeny (Arribere et al. 2014). (B and C) A depiction of the loci, mutant alleles, PAMs, sgRNAs, and donor oligonucleotides used to demonstrate the capability of the VQR and VRER variants to perform genome editing in C. elegans. Initiating an editing event, a double-strand break is made by a Cas9 variant (marked with scissors). The break is repaired through recombination with a homologous donor template carrying a mutation (in green) that confers a dominant phenotype and additional silent mutations (in yellow) that prevent repeated cleavage. Numbers of viable animals following injection and numbers of broods with successful HR events are shown; as with other microinjection studies in this system (e.g., Arribere et al. 2014), we note that raw success rates can show considerable variation between injection sets, suggesting that such frequencies be taken as illustrative rather than comparative.
Editing of an endogenous PAM allows a direct comparison of three Cas9 transgenes using a single sgRNA target
We used a CRISPR/Cas9 genome editing approach (Arribere et al. 2014) to build a set of target sites at an endogenous genomic location that were designed to allow parallel evaluation of efficacy for the wild-type, VQR, and VRER proteins. The new alleles were engineered with synonymous substitutions in dpy-10 that convert an endogenous NGA PAM to either NGG or NGCG (Figure 3A) . The strains, in conjunction with their wild-type ancestor, allowed the same sgRNA expression vector to be used with all three Cas9 transgenes (wild-type, VQR, and VRER) to introduce the dpy-10(cn64) mutation by HR with the donor template (Figure 3B). Therefore, we eliminated the variables of sgRNA sequence and distance of the marker mutation from the site of Cas9 cleavage. In this controlled assay, the effectiveness of VQR and wild-type Cas9 was comparable, while the VRER Cas9 exhibited lower efficiency (Figure 3, C and D).
Figure 3.
Direct comparison of Cas9s recognizing three different PAMs using a single sgRNA by editing a C. elegans genomic target. (A) The endogenous NGA PAM used in the conversion of dpy-10 by the VQR variant was edited to be an NGG or an NGCG PAM using mutations that did not alter the DPY-10 protein sequence. The resulting strains allowed a single sgRNA construct to be employed to target dpy-10 for conversion with the three Cas9 transgenes (wild-type, VQR, and VRER). (B) Each Cas9 transgene was injected into a strain with its cognate PAM present in dpy-10 adjacent to the sgRNA target. The break induced by each Cas9 using this sgRNA (marked with scissors) was the same distance from the mutated base that conferred the Rol phenotype. (C) The wild-type Cas9 and VQR variants were both highly competent to produce Rol progeny, with many injected animals producing “jackpots” (>10 Rol progeny). The VRER Cas9 induced Rol animals through HR at a significantly lower frequency than the other two Cas9s. (D) In addition to Rol progeny, the injected worms generally yielded as many or more Dpy and Dpy Rol progeny, which derived largely from indels introduced by the NHEJ repair pathway but also could result from NHEJ repair on one chromosome and HR repair on the other. The number of injected animals with >10 Dpy or Dpy Rol progeny is explicitly indicated in the plots for the wild-type and VQR Cas9s, where that outcome was very frequent. The VRER Cas9, while producing fewer Rol progeny than wild-type Cas9 or the VQR variant (30 of 35 injected animals had 0 Rol progeny), displayed considerable activity, evidenced by the number of Dpy Rol and Dpy animals observed.
Co-conversion and PAM specificity of wild-type and mutant Cas9s
The VQR and VRER Cas9 mutants differ only slightly from the wild-type protein; therefore, we considered an evaluation of their PAM preferences to be important. To characterize PAM stringency, we used the dpy-10(cn64) and unc-58(e665) phenotypes to assay for HR events. We injected a series of six mixtures that contained donor oligonucleotides for the dpy-10(cn64) and unc-58(e665) marker mutations. The injection mixes also all included two sgRNA expression vectors, one targeting a sequence in unc-58 with an NGG PAM and one targeting a sequence in dpy-10 with either an NGA or an NGCG PAM (Figure 4A).
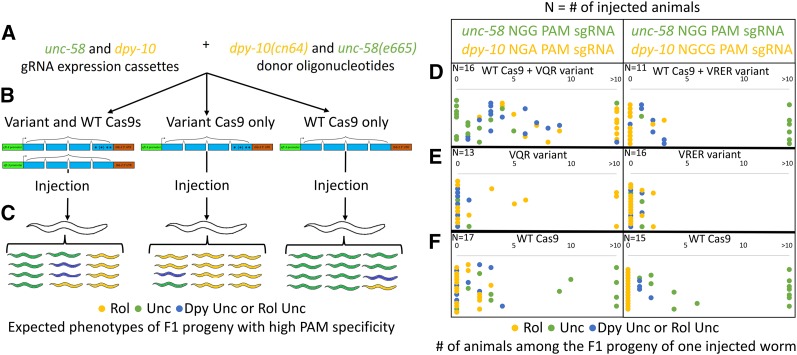
Figure 4.
Co-conversion by each Cas9 mutant and wild-type Cas9 demonstrates PAM specificity. (A) A schematic of the reagents injected to assess PAM stringency. dpy-10(cn64) and unc-58(e665) donor oligonucleotides were included in all injection mixes, as well as sgRNA vectors with a target in unc-58 with an NGG PAM. A sgRNA vector with a target in dpy-10 with an NGA or an NGCG PAM was present if the VQR or VRER Cas9 variant was to be tested, respectively. (B) One variant (VQR or VRER) Cas9 transgene and the wild-type Cas9 transgene were injected together or individually, always co-injected with both donor oligonucleotides and sgRNA expression vectors. (C) The VQR and VRER variants should cleave dpy-10 and produce Rol progeny in the F1 generation, while the wild-type Cas9 should cleave the unc-58 locus and produce Unc progeny. An abundance of both Rol and Unc phenotypes was expected when variant and wild-type Cas9s were present, in addition to Dpy Unc and Rol Unc animals with mutations at both loci. When only one Cas9 transgene was included in the injection mix, a preponderance of the corresponding phenotype (Rol for a variant and Unc for wild-type) should result if the Cas9s were specific to their PAMs. (D) When either variant transgene was injected with the wild-type Cas9 transgene, the progeny exhibited an abundance of Rol, Dpy Unc, Rol Unc, and Unc phenotypes, indicating robust activity at both loci. (E) When the wild-type transgene was not included, Rol progeny were a substantial fraction, although some Dpy Unc, Rol Unc, and Unc animals were observed. Worms injected with the VRER Cas9 variant alone produced relatively few Rol animals, but additional Dpy Rol and Dpy worms were present among their progeny (Figure S1). Many injected animals had no progeny with a given mutant phenotype. For the VQR transgene, these counts were 6 (0 Rol), 12 (0 Unc), and 12 (0 Dpy Unc or Rol Unc). For the VRER transgene, these counts were 12 (0 Rol), 14 (0 Unc), and 14 (0 Dpy Unc or Rol Unc). (F) When the wild-type Cas9 alone was injected, the result was an anticipated excess of Unc convertant progeny, but a small number of animals with Dpy Unc or Rol Unc phenotypes also were present. Many injected animals had no progeny with a given mutant phenotype. When the wild-type transgene was injected with an NGA PAM sgRNA expression vector, these counts were 10 (0 Rol), 7 (0 Unc), and 12 (0 Dpy Unc or Rol Unc). When the wild-type transgene was injected with an NGCG PAM sgRNA expression vector, these counts were 15 (0 Rol), 2 (0 Unc), and 11 (0 Dpy Unc or Rol Unc).
For each of the Cas9 variants, three mixes were injected. In one, both the variant (VQR or VRER) and the wild-type Cas9 transgenes were present, while in the other two, only one type was included (Figure 4B). If the proteins discriminated effectively between their preferred PAMs, the expectation when both transgenes were present in the injection mix was to observe an abundance of both Rol and Unc phenotypes in the F1 progeny. We also predicted the presence of Dpy Unc and Rol Unc animals with mutations at both loci (resulting from NHEJ or HR at dpy-10 and HR at unc-58) (Figure 4C). When the wild-type Cas9 was omitted from the injection mix, we expected to observe a majority of Rol animals (Figure 4C). Conversely, in the absence of either Cas9 variant, we anticipated a preponderance of Unc animals (Figure 4C).
We found that each of the classes of homologous recombinants was most efficiently recovered in experiments in which the matched Cas9 was present (Figure 4, D–F). Leaving out the cognate Cas9 resulted in a consistent and dramatic decrease in insertion activity (Figure 4, E and F). Selectivity, although high, was not without exception. The VQR and VRER variants could occasionally promote HR with an NGG PAM (Figure 4E). Wild-type Cas9 could induce HR with an NGA PAM (albeit rarely) and also exhibited a low level of activity using an NGCG PAM (Figure 4F). These results are consistent with previous observations of strong but not perfect PAM dependence for both wild-type Cas9 (Hsu et al. 2013; Pattanayak et al. 2013; Fu et al. 2014; Wu et al. 2014) and the VQR/VRER variants (Kleinstiver et al. 2015).
Editing of an endogenous PAM elucidates the cleavage efficiency of the VQR variant with different NGAN PAMs
Kleinstiver et al. (2015) reported differential effectiveness of the VQR variant depending on the base following the NGA PAM. Their description of the VQR variant summarized the influence as NGAG > NGAA = NGAT > NGAC, with NGAA and NGAT having approximately 75% of the effectiveness of NGAG, while NGAC was about 50% effective. When converting dpy-10 and unc-58, we used NGAG and NGAA PAMs, respectively, and observed much greater efficiency with NGAG. However, the contributing factors of distinct sgRNA sequences, different distances of marker mutations from the Cas9 cleavage site, and disparate genomic locations underscored the value of a more controlled evaluation. We therefore developed a common sgRNA assessment strategy similar to the means by which we compared the three Cas9 variants. Using CRISPR/Cas9, we edited the genome near the site of the dpy-10(cn64) mutation (Arribere et al. 2014), creating four different strains. In each, an NGG PAM adjacent to a sgRNA target we had previously verified with wild-type Cas9 was converted to four different NGAN PAMs with each of the four possible nucleotides at the fourth position. This approach allowed us to use a single proven sgRNA to accurately test the VQR Cas9 cleavage efficiency employing each of the NGAN PAMs (Figure 5A) . The modifications involved making a nonsynonymous substitution converting an alanine codon to four different serine codons (all the resulting Ala → Ser variants were wild-type in phenotype).
Figure 5.
Direct comparison of cleavage efficiency by the VQR Cas9 with a single sgRNA using the four NGAN PAMs by editing a C. elegans genomic target. (A) A genomic target sequence with an NGG PAM used previously to convert dpy-10 as a marker for Cas9 expression (Arribere et al. 2014) was edited by CRISPR/Cas9 to make an alanine-to-serine substitution (CCG to TCG), alter the wobble position of the upstream valine codon (GTN), and generate all four NGAN PAMs. (B) The NGAG PAM was highly efficient in recruiting the VQR Cas9 to convert dpy-10 using this sgRNA, with most injected worms yielding >10 Rol progeny. The NGAA and NGAT PAMs also were able to induce conversion, but to a more modest extent. The NGAC PAM was significantly less proficient but still competent. Wild-type worms with an NGG PAM injected with the wild-type Cas9 transgene produced a number of Rol progeny comparable to the NGAG strain injected with the VQR variant transgene. The number of injected animals with >10 Rol progeny is explicitly indicated in the plots where that outcome was very frequent.
We observed that injected animals derived from all four strains gave rise to Rol progeny, confirming effective HR using each of the NGAN PAMs. Our results strongly support NGAG as the most effective PAM and substantiate the report that NGAA and NGAT are both more effective than NGAC. When injected with the wild-type Cas9 transgene, wild-type animals with an NGG PAM adjacent to the same sgRNA target produced Rol progeny in quantities similar to worms injected with the VQR transgene with an NGAG PAM in the same position (Figure 5B).
Conclusions
We have demonstrated that precise genome engineering can be extended to a broader range of target loci in the C. elegans genome by using two Cas9 transgenes expressing modified proteins with alternative PAM specificities. We undertook a careful comparison of the altered Cas9 proteins to the wild-type by editing a PAM in a genomic target, dpy-10. Our engineering permitted experimental comparisons in which a single sgRNA could be used in multiple tester strains in which its constant target sequence was adjacent to the PAM variants recognized by the different Cas9s. Such assays showed that the VQR Cas9, when targeted to an endogenous NGAG PAM, can promote HR at levels comparable to wild-type Cas9. We similarly probed the performance of the VQR variant by editing another PAM in dpy-10 to test the effect of the downstream base. Our results provided corroboration of a gradation in activity (Kleinstiver et al. 2015), with NGAC at this site being the least efficient and NGAG being the most effective, extending these results to a meiotic system and to C. elegans. This work demonstrates the potential of genome engineering to allow efficient and accurate testing of Cas9 transgenes and characterization of their PAM requirements. In addition, the reagents we developed can serve as a foundation to generate C. elegans expression vectors for Cas9 fusion proteins with alternate PAM recognition.
C. elegans has an AT-rich genome composition, leading to frequent challenges in identifying NGG PAMs with suitable proximity for efficient oligonucleotide-templated editing. The availability of reagents that allow the use of alternative NGA PAMs should greatly expand the regions of the genome accessible to efficient editing by such methods. Examining coding sequences (Figure S2, A and B) and 3’ UTRs (Figure S2, C and D), we indeed see substantial expansion of the targetable repertoires. For example, the addition of sequences within five bases of NGA PAM cleavage sites to those within five bases of NGG cleavage sites would expand the number of efficiently targetable bases in coding sequence by 44% and more than double the number in 3’ UTR sequence.
Acknowledgments
We thank Nimit Jain, Christian Frøkjær-Jensen, Joshua Arribere, Elif Cenik, Karen Artiles, and Massa Shoura for their critical reading of the manuscript, as well as every member of the Fire Laboratory for their many helpful suggestions during the course of this work. In particular, we thank Joshua Arribere, who developed the oligonucleotide-templated CRISPR/Cas9 HR protocol, which was the foundation for these experiments, and Christian Frøkjær-Jensen for Gibson cloning advice and suggesting the use of a single sgRNA vector to test different Cas9 variants. This work was supported by National Institutes of Health grants R01-GM37706, T32-GM007790, and T32-HG000044.
Footnotes
Communicating editor: O. Hobert
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.185041/-/DC1.
Literature Cited
- Anders C., Niewoehner O., Duerst A., Jinek M., 2014. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X. H., Artiles K. L., Hartman P. S., et al. , 2014. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., et al. , 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo J. E., Norville J. E., Mali P., Rios X., Aach J., et al. , 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41: 4336–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., Goldstein B., 2015. Streamlined genome engineering with a self-excising drug selection cassette. Genetics 200: 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt K. M., Mali P., Braff J. L., Moosburner M., Yaung S. J., et al. , 2013. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat. Methods 10: 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., 2005. Nucleic acid structure and intracellular immunity: some recent ideas from the world of RNAi. Q. Rev. Biophys. 38: 303–309. [DOI] [PubMed] [Google Scholar]
- Friedland A. E., Tzur Y. B., Esvelt K. M., Colaiácovo M. P., Church G. M., et al. , 2013. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10: 741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B. X. H., Hansen L. L., Artiles K. L., Nonet M. L., Fire A. Z., 2014. Landscape of target:guide homology effects on Cas9-mediated cleavage. Nucleic Acids Res. 42: 13778–13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R.-Y., Venter J. C., Hutchison C. A., et al. , 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6: 343–345. [DOI] [PubMed] [Google Scholar]
- Gilbert L. A., Larson M. H., Morsut L., Liu Z., Brar G. A., et al. , 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., et al. , 2013. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton I. B., D’Ippolito A. M., Vockley C. M., Thakore P. I., Crawford G. E., et al. , 2015. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. D., Scott D. A., Weinstein J. A., Ran F. A., Konermann S., et al. , 2013. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C. H., Friedman R. C., Ruby J. G., Bartel D. P., 2011. Formation, regulation and evolution of Caenorhabditis elegans 3[prime]UTRs. Nature 469: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns N. A., Pham H., Tabak B., Genga R. M., Silverstein N. J., et al. , 2015. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat. Methods 12: 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ishidate T., Ghanta K. S., Seth M., Conte D., et al. , 2014. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 197: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstiver B. P., Prew M. S., Tsai S. Q., Topkar V. V., Nguyen N. T., et al. , 2015. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. D., Yang J., Kramer J. M., 1993. Molecular and genetic analyses of the Caenorhabditis elegans dpy-2 and dpy-10 collagen genes: a variety of molecular alterations affect organismal morphology. Mol. Biol. Cell 4: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-F., Norville J. E., Aach J., McCormack M., Zhang D., et al. , 2013. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31: 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Guo H., Yao D., Xiong K., Li Y., et al. , 2015. Regulation of transcriptionally active genes via the catalytically inactive Cas9 in C. elegans and D. rerio. Cell Res. 25: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Aach J., Stranges P. B., Esvelt K. M., Moosburner M., et al. , 2013. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Folkmann A., Rasoloson D., Seydoux G., 2015. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR/Cas9 ribonucleoprotein complexes. Genetics 201: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Wang Y., Smith H., Lee C.-Y. S., Calidas D., et al. , 2014. Scalable and versatile genome editing using linear DNAs with micro-homology to Cas9 sites in Caenorhabditis elegans. Genetics 198: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V., Lin S., Guilinger J. P., Ma E., Doudna J. A., et al. , 2013. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 31: 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F. A., Cong L., Yan W. X., Scott D. A., Gootenberg J. S., et al. , 2015. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redemann S., Schloissnig S., Ernst S., Pozniakowsky A., Ayloo S., et al. , 2011. Codon adaptation-based control of protein expression in C. elegans. Nat. Methods 8: 250–252. [DOI] [PubMed] [Google Scholar]
- Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., et al. , 2013. The Million Mutation Project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. D., 2015. Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair. Genetics 199: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kriz A. J., Sharp P. A., 2014. Target specificity of the CRISPR-Cas9 system. Quant. Biol. 2: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Jaenisch R., 2014. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9: 1956–1968. [DOI] [PubMed] [Google Scholar]
- Zhao P., Zhang Z., Ke H., Yue Y., Xue D., 2014. Oligonucleotide-based targeted gene editing in C. elegans via the CRISPR/Cas9 system. Cell Res. 24: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]