Figure 7.
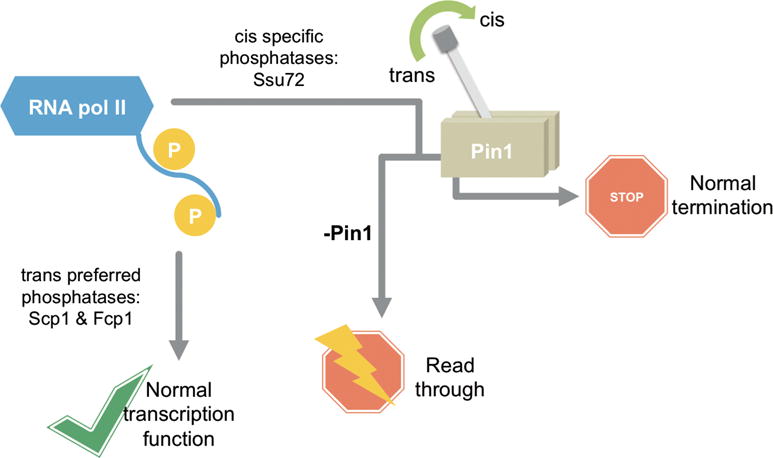
Model of differentiated regulation mediated by proline isomerization of CTD in RNA polymerase II dephosphorylation. Trans-preferred or specific phosphatases, Scp1 and Fcp1, have substrate consistently available due to the thermodynamic preference for trans-proline in the CTD. Therefore, these enzymes bypass regulation by prolyl isomerases like Pin1. However, cis-specific phosphatases like Ssu72 rely on a minor substrate pool containing the cis-proline isomer and quickly deplete their available substrate. Prolyl isomerases, like Pin1, can restore the equilibrium between cis and trans isomers and replenish substrate pools. This regulatory switch provides for proper RNA polymerase II CTD phosphorylation levels and normal transcription termination. Upon Pin1 disruption or knockdown, global transcription defects, like read-through, may occur.
