Abstract
We investigated the effects of dietary zinc deficiency on oxidative stress and bone metabolism. Four-week-old male Wistar rats were randomly assigned to one of three groups for 4 weeks: a zinc-adequate group (30 ppm); a zinc-deficient group (1 ppm); and a pair-fed group (30 ppm) that was pair-fed to the zinc-deficient group. The iron content and the thiobarbituric acid reactive substance level in bone were higher in the zinc-deficient group than in the zinc-adequate and pair-fed groups. The mRNA expression level of osteoblastogenesis-related genes such as bone morphogenetic protein 2 and runt-related transcription factor 2 was lower in the zinc-deficient group than in the zinc-adequate and pair-fed groups. In contrast, the mRNA expression levels of tumor necrosis factor-α, interleukin-1β and osteoclastogenesis-related genes such as receptor activator of nuclear factor-κB ligand and nuclear factor of activated T cells cytoplasmic 1 were higher in the zinc-deficient group than in the zinc-adequate and pair-fed groups.
These findings suggested that dietary zinc deficiency reduced osteoblastogenesis via a decrease in the expression of bone morphogenetic protein 2 and increased osteoclastogenesis via enhancement of the expression of receptor for activator of nuclear factor-κB ligand induced by oxidative stress-stimulated tumor necrosis factor-α and interleukin-1β.
Keywords: zinc deficiency, RANKL, oxidative stress, iron, cytokine
Introduction
Zinc is an essential trace metal and serves functions in catalysis or structural stabilization of over 300 enzymes. Zinc deficiency has been observed in disorders of the Crohn’s disease, renal diseases and liver disease.(1,2) Moreover, it is known that zinc deficiency leads to growth retardation, dermatitis, depilation and dysfunction of reproductive systems.(3) Zinc has been considered an important factor in bone metabolism since bone contains approximately 30% of the zinc in the body. Zinc has been known to promote bone formation and inhibit bone resorption in in vitro studies.(4,5) In a previous study, we demonstrated that dietary zinc deficiency decreased bone formation and increased bone resorption in rats.(6)
Zinc deficiency can lead not only to growth retardation, but also to increased oxidative stress and the generation of inflammatory cytokines.(7,8) It is also a cofactor of super oxide dismutase (SOD), an enzyme that plays a key role in the protection against oxidative tissue injury. In addition, several studies have reported that dietary zinc deficiency increased thiobarbituric acid reactive substance (TBARS) levels in testicle, kidney, brain and lung.(9–11) A number of other studies have also indicated that dietary zinc deficiency lead to the accumulation of iron in several tissues, such as testes and liver.(12–14) Iron is involved in a series of single-electron transfer reactions that can generate reactive oxygen species (ROS), which may lead to tissue damage as a consequence of lipid, nucleic acid and/or protein oxidative damage.(15,16) Moreover, it has been demonstrated that oxidative stress increased osteoclast numbers and activity in in vitro and in vivo studies.(17) Therefore, we predict that a decrease in dietary zinc induces the accumulation of iron and enhances oxidative damage in bone, leading to an increase in bone resorption, which is a factor that can promote bone fractures. However, there have been no studies examining the effects of dietary zinc deficiency on oxidative stress and iron level in bone.
In this study, we attempted to clarify whether dietary zinc deficiency leads to an increase in oxidative stress in bone, and subsequently, how this increase may affect abnormal bone development. Therefore, we conducted two experiments in this study: the first experiment investigated the effects of dietary zinc deficiency on oxidative stress in bone, and the second experiment investigated the effects of dietary zinc deficiency on the expression levels of bone metabolism- and oxidative stress-related genes in bone.
Methods
Experiment I
Four-week-old male Wistar rats (Clea Japan, Tokyo, Japan) were kept in individual stainless steel wire-bottom cages in a temperature-, humidity- and light-controlled room (22°C, 55% humidity, 12-h light-dark cycle). After a 3-day adaptation period with control diet (30.0 mg Zn/kg), the rats were randomly divided into three groups consisting of 8 rats in each on body weight. For the experiment, the rats in two of the three groups had free access to a control diet (C group) or a zinc-deficient diet (ZD group). The rats in the third group (PF group) were pair-fed with the control diet to the mean intake of the ZD group. The experimental diets were based on the AIN-93G diet(18) with egg albumin as the protein source (Table 1). The zinc-deficient diet was prepared from a basal diet with the addition of a zinc-free AIN-93G mineral mixture instead of the AIN-93G mineral mixture. The zinc concentrations of the control and zinc-deficient diets were 30.0 and 1.0 ppm, respectively.
Table 1.
Composition of the experimental diets
| Ca | ZDb | |
|---|---|---|
| Chemical analysis Zn level (%) | 0.003 | 0.0001 |
| Ingredient | g/1 kg diet | |
| Egg albumin | 200.0 | 200.0 |
| Corn starch | 529.486 | 529.486 |
| Sucrose | 100.0 | 100.0 |
| Soybean oil | 70.0 | 70.0 |
| Cellulose powder | 50.0 | 50.0 |
| AIN-93G mineral mixture | 35.0 | 35.0c |
| AIN-93 vitamin mixture | 10.0 | 10.0 |
| l-cystine | 3.0 | 3.0 |
| Choline bitartrate | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 |
Prepared according to the AIN-93G formulation. aControl diet. bZinc deficient diet. cThe mineral mixture is a modification of AIN-93G mineral mixture without zinc carbonate.
After the 4-week experiment, the rats were fasted for 12 h and were then anesthetically sacrificed for dissection; blood and femur samples were collected for analysis. The blood samples were centrifuged at 3,000 rpm for 15 min to obtain plasma. Serum samples were stored at −80°C until analysis. The right femur was immediately frozen in liquid N2 and stored at −80°C for the measurement of TBARS levels, and the left femur was removed and stored in 70% ethanol for subsequent bone mineral density (BMD) and mineral content analyses.
Experiment II
As in Experiment I, the rats were acclimatized and divided into three groups on the same day, and the composition of the diets and the experimental groups were also the same. As described in Experiment I, after the 4-week experiment, the rats were fasted for 12 h and were then anesthetically sacrificed for dissection; blood and femur samples were collected for analysis. The femur was immediately frozen in liquid N2 and stored at −80°C for total RNA extraction.
These studies were approved by the Animal Studies Committee of Tokyo University of Agriculture, and all procedures involving rats were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Tokyo University of Agriculture.
Biochemical analysis
Serum zinc was measured using a metallo-assay kit (Metallogenics, Chiba, Japan). Serum calcium and zinc, and bone calcium and iron in femur were analyzed using atomic absorption spectrophotometry (ZA3300, Hitachi, Ltd., Tokyo, Japan) according to the method of Gimblet et al.(19) Bone phosphorus was analyzed colorimetrically according to the method of Gomori.(20) Serum Osteocalcin concentration was measured using an Osteocalcin Rat ELISA System (GE Healthcare UK Ltd., Amersham, England). The serum C-terminal telopeptide of type I collagen (CTx) level was assayed using the RatLaps ELISA (Immunodiagnostic Systems Nordic A/S, Herlev, Denmark).
Radiographic analysis of the femur
Femurs were removed from each rat and were stored at 5°C until analysis. Bone mineral content (BMC; mg), bone area (BA; cm2) and BMD (mg/cm2), which was calculated by dividing BMC by BA, of the left femur of each rat were measured using dual-energy X-ray absorptiometry (DXA; DCS-600EX-R; Aloka, Tokyo, Japan).
TBARS measurement of the femur
The TBARS levels in the femurs were estimated by the methods of Kikugawa et al.(21) with the modification of Ohkawa et al.(22) The femur samples were homogenized with a 1.15% potassium chloride solution mixed with 0.8% butylated hydroxytoluene, 8.1% sodium dodecyl sulfate and 20% acetic acid adjusted to pH 3.5 with 10 N sodium hydroxide, 5 mM ethylenediamineteraacetic acid and 0.8% thiobarbituric acid. The mixture was kept at exactly 5°C for 60 min and was then heated at 100°C for 60 min. After cooling, the mixture was extracted with water and n-butanol:pyridine (15:1, v/v). The mixture was centrifuged at 3,000 rpm for 10 min, and the absorbance was measured at 532 nm by a spectrophotometer (U-2000 HITACHI, Tokyo, Japan). The level of TBARS was expressed as nmol/g protein in bone. The protein content of the homogenized femurs was determined using a Protein Assay Rapid Kit (Wako, Osaka, Japan).
Glutathione (GSH), glutathione reductase (GR) and glutathione peroxidase (GPx) measurements of the femur
The GSH, GR and GPx levels were measured using a Glutathione Assay Kit, a Glutathione Reductase Activity (Microplate Assay) Kit and a Glutathione Peroxidase Assay Kit (all from Northwest Life Science Specialties LLC, Vancouver, WA), respectively. The femur samples were homogenized with a 1.15% potassium chloride solution. The levels of GSH, GR and GPx were expressed as µmol/g protein, mU/mg protein and mU/mg protein, respectively.
RNA extraction and quantitative RT-PCR
Total RNA was isolated from the right femur using TRIzol Reagent (Invitrogen, Carlsbad, CA) and was purified using the RNeasy Mini Kit (QIAGEN K.K., Tokyo, Japan). DNase digestion was performed using the RNase-Free DNase Set (QIAGEN K.K.). The quality and quantity of total RNA were assayed using agarose gel electrophoresis and a Nano Drop 2000c (Thermo Fisher Scientific, Waltham, MA), respectively. Complementary DNA (cDNA) was synthesized using the Prime Script RT Reagent Kit (Takara Bio Inc., Shiga, Japan). For real-time PCR, reaction mixture was prepared using the TaqMan Gene Expression Master Mix (Applied Biosystems, Foster, CA) for TaqMan Gene Expression Assays (Applied Biosystems) for the following rat genes: osteoprotegerin (OPG) (Assay ID: Rn00563499_m1), collagen type Iα1 (Col1α1) (Assay ID: Rn01463848_m1), bone morphogenetic protein 2 (BMP2) (Assay ID: Rn00567818_m1), runt-related transcription factor 2 (Runx2) (Assay ID: Rn01512298_m1), Osterix (Assay ID: Rn02769744_s1), Osteocalcin (Assay ID: Rn00566386_g1), alkaline phosphatase (ALP) (Assay ID: Rn01516028_m1), tumor necrosis factor-α (TNFα) (Assay ID: Rn01525859_g1), interleukin-1β (IL1β) (Assay ID: Rn00580432_m1), receptor for activator of NF-κB ligand (RANKL) (Assay ID: Rn00589289_m1), c-Fos (Assay ID: Rn00487426_g1), tumor necrosis factor receptor-associated factor 6 (TRAF6) (Assay ID: Rn01512911_m1), tumor necrosis factor receptor-associated factor 2 (TRAF2) (Assay ID: Rn01758426_m1), nuclear factor of activated T cells cytoplasmic 1 (NFATc1) (Assay ID: Rn04280453_m1), cathepsin K (CTSK) (Assay ID: Rn00580723_m1), tartrate-resistant acid phosphatase (TRAP) (Assay ID: Rn00569608_m1), macrophage colony-stimulating factor (M-CSF) (Assay ID: Rn00696122_m1), macrophage colony-stimulating factor receptor (c-Fms) (Assay ID: Rn01491957_m1), heme oxygenase 1 (HO-1) (Assay ID: Rn01536933_m1), CuZn-superoxide dismutase (Cu/Zn-SOD) (Assay ID: Rn00566938_m1), Mn-SOD (Assay ID: Rn00690588_g1), glutathione reductase (GR) (Assay ID: Rn01482159_m1), glutathione peroxidase (GPx) (Assay ID: Rn00577994_g1) and β-actin (Assay ID: Rn00667869_m1). Real-time PCR was performed using a StepOne Real-Time PCR System (Applied Biosystems). The levels of gene transcripts in each sample were determined using the relative standard curve method and are expressed as a ratio relative to β-actin mRNA, with the level of the C group set to 1.
Statistics
Data are expressed as the mean ± standard error of the mean (SEM). The significance of differences between groups was determined using one-way analysis of variance and Bonferroni’s multiple-comparison tests. The correlation between iron content and TBARS level in bone was determined using Pearson correlation coefficients. Differences were considered significant at p<0.05.
Results
Experiment I
Final body weight and serum mineral concentrations.
The final body weight was significantly lower in the PF and ZD groups than in the C group, with no difference between the PF and ZD groups (Table 2). The serum zinc and calcium concentrations were significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Table 2.
The effect of dietary zinc deficiency on serum mineral levels, bone parameters, oxidative stress and antioxidant indicators
| C | PF | ZD | |
|---|---|---|---|
| Body weight (g) | |||
| Initial | 84.24 ± 0.91a | 84.32 ± 0.84a | 84.86 ± 0.89a |
| Final | 240.95 ± 3.71a | 146.43 ± 1.52b | 149.38 ± 1.97b |
| Serum | |||
| Zinc (µM) | 24.56 ± 1.03a | 24.54 ± 0.74a | 9.18 ± 0.62b |
| Calcium (mM) | 2.92 ± 0.04a | 2.99 ± 0.06a | 2.52 ± 0.04b |
| Osteocalcin (ng/ml) | 106.97 ± 3.76a | 103.49 ± 4.95a | 58.46 ± 3.50b |
| CTx (ng/ml) | 64.96 ± 0.94a | 60.25 ± 0.92a | 82.85 ± 3.57b |
| Femur | |||
| Length (cm/100 g body weight) | 1.30 ± 0.02a | 2.08 ± 0.03b | 2.02 ± 0.02b |
| Weight (g/100 g body weight) | 0.15 ± 0.002a | 0.22 ± 0.003b | 0.21 ± 0.004b |
| BA (cm2) | 1.69 ± 0.02a | 1.60 ± 0.01b | 1.59 ± 0.02b |
| BMC (mg) | 172.30 ± 2.78a | 154.20 ± 1.56b | 145.29 ± 3.64b |
| BMD (mg/cm2) | 101.75 ± 0.90a | 96.53 ± 1.35a | 85.93 ± 2.56b |
| Zinc (µg/cm2) | 86.70 ± 1.09a | 86.61 ± 1.80a | 21.60 ± 1.22b |
| Calcium (mg/cm2) | 41.91 ± 0.57a | 39.79 ± 0.71a | 36.23 ± 0.48b |
| Phosphorus (mg/cm2) | 19.98 ± 0.49a | 18.89 ± 0.43a | 17.32 ± 0.33b |
| Iron (µg/cm2) | 9.45 ± 0.50a | 9.82 ± 0.35a | 21.20 ± 2.94b |
| TBARS (nmol/g protein) | 152.50 ± 7.02a | 152.34 ± 8.43a | 188.69 ± 9.36b |
| GSH (µmol/g protein) | 9.34 ± 0.80a | 9.54 ± 0.96a | 8.91 ± 0.80a |
| GR (mU/mg protein) | 23.28 ± 2.11a | 22.63 ± 1.93a | 21.78 ± 2.02a |
| GPx (mU/mg protein) | 8.55 ± 1.51a | 13.02 ± 5.43a | 10.85 ± 1.97a |
Values are expressed as means ± SEM for each group. a,b,cValues with different superscript letters are significantly different, p<0.05.
Bone parameters.
The length and weight of the femur were significantly higher in the PF and ZD groups than in the C group, with no difference between the PF and ZD groups (Table 2). The BA and BMC of the femur were significantly lower in the PF and ZD groups than in the C group, with no difference between the PF and ZD groups. BMD was significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
The serum Osteocalcin concentration was significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups. The serum CTx concentration was significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Bone mineral contents.
The zinc, calcium and phosphorus contents of the femur were significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Table 2). On the other hand, the iron content of the femur was significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Bone lipid peroxidation (TBARS), GSH, GR and GPx.
The TBARS level of the femur was significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Table 2). The GSH, GR and GPx levels of the femur were not significantly different between any of the groups.
The correlation between iron content and TBARS level.
The iron content of femur was positively correlated with the TBARS level in femur (r = 0.762; p<0.001).
Experiment II
Final body weight and serum mineral concentrations.
Similar to the results of Experiment I, the final body weight was significantly lower in the PF and ZD groups than in the C group, with no difference between the PF and ZD groups (Table 3). The serum zinc and calcium concentrations were significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Table 3.
Effect of dietary zinc deficiency on serum mineral levels and markers of bone metabolism
| C | PF | ZD | |
|---|---|---|---|
| Body weight (g) | |||
| Initial | 83.46 ± 1.00a | 83.40 ± 0.92a | 83.41 ± 0.89a |
| Final | 241.89 ± 5.56a | 146.16 ± 0.97b | 142.23 ± 4.20b |
| Serum | |||
| Zinc (µM) | 23.17 ± 1.08a | 20.34 ± 0.73a | 7.78 ± 0.48b |
| Calcium (mM) | 3.00 ± 0.09a | 2.81 ± 0.10a | 2.46 ± 0.07b |
| Osteocalcin (ng/ml) | 105.35 ± 8.47a | 89.40 ± 3.53a | 53.84 ± 4.23b |
| CTx (ng/ml) | 75.49 ± 2.87a | 64.43 ± 2.91a | 89.16 ± 3.58b |
Values are expressed as means ± SEM for each group. a,b,cValues with different superscript letters are significantly different, p<0.05.
Markers of bone turnover.
Similar to the results of Experiment I, the serum Osteocalcin concentration was significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Table 3). The serum CTx concentration was significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Quantitation of mRNA expression in the femur.
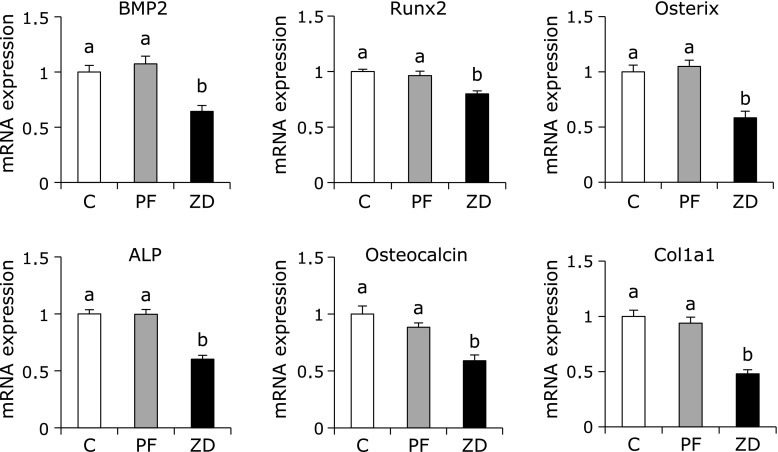
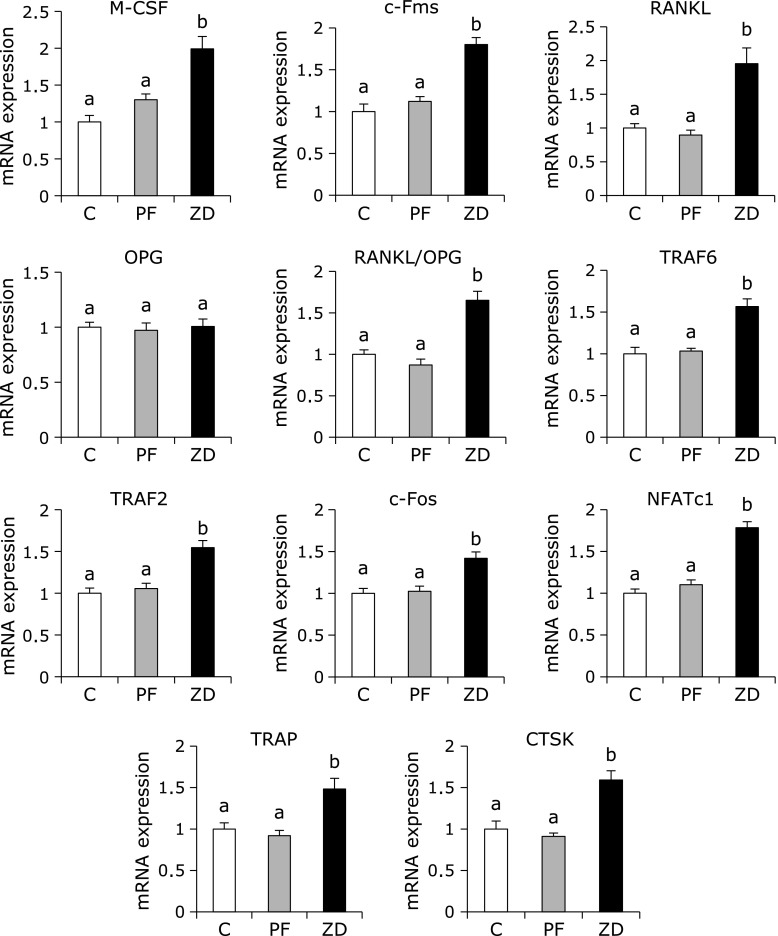
The mRNA levels of BMP2, Runx2, Osterix, ALP, Osteocalcin and Col1a1 were significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Fig. 1). In contrast, the mRNA levels of M-CSF, c-Fms, RANKL, TRAF6, TRAF2, c-Fos, NFATc1, TRAP and CTSK were significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Fig. 2). The mRNA expression level of OPG was not significantly different between any of the groups, while the RANKL/OPG ratio was significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups.
Fig. 1.
Effect of dietary zinc deficiency on the gene expression levels of osteoblastogenesis-related factors and osteoblast-specific proteins in femur. The mRNA expression levels of BMP2, Runx2, Osterix, ALP, Osteocalcin and Col1a1 were determined using qRT-PCR. The ordinate axis indicates the relative amount of mRNA compared with that from the C group. Gene expression levels were normalized to the levels of β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
Fig. 2.
Effect of dietary zinc deficiency on the gene expression levels of osteoclastogenesis-related factors and osteoclast-specific proteins in femur. The mRNA expression levels of M-CSF, c-Fms, RANKL, OPG, TRAF6, TRAF2, c-Fos, NFATc1, TRAP and CTSK were determined using qRT-PCR. The RANKL/OPG ratio was also calculated. The ordinate axis indicates the relative amount of mRNA compared with that from the C group or compared with the level of OPG mRNA (for the RANKL/OPG ratio). Gene expression levels were normalized to the levels of β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
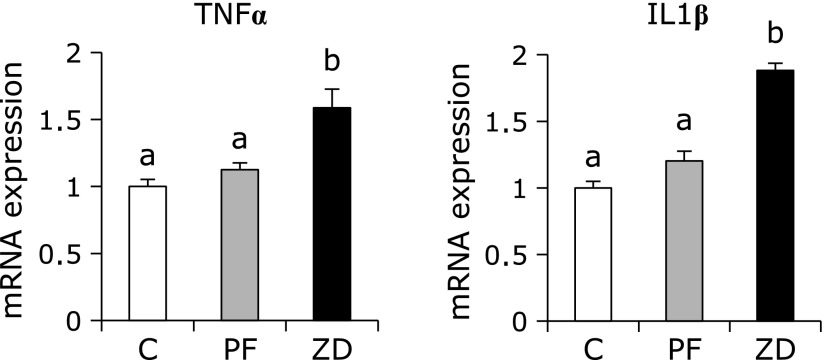
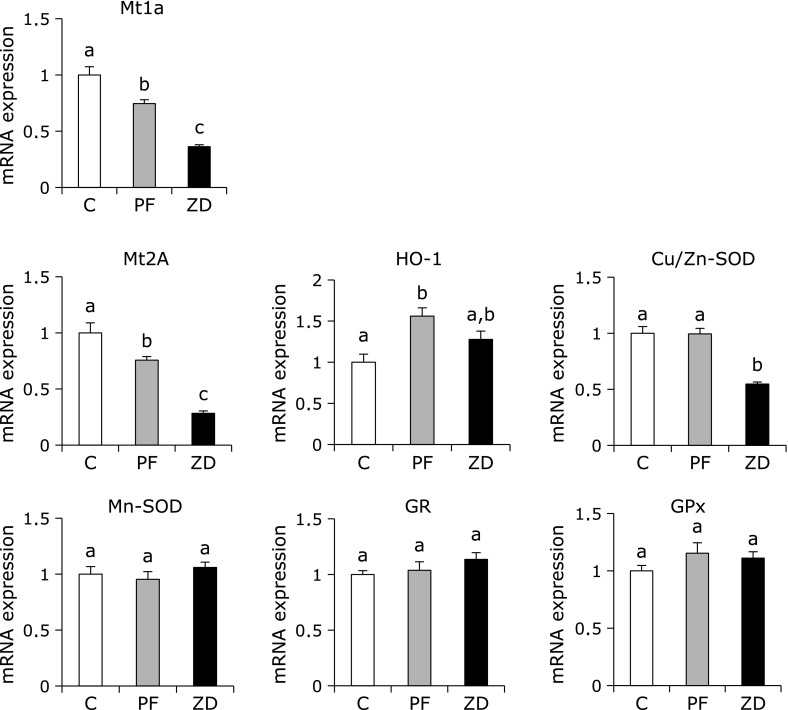
The gene expression levels of the cytokines TNFα and IL1β were significantly higher in the ZD group than in the C and PF groups, with no difference between the C and PF groups (Fig. 3). The mRNA expression levels of Mt1a and Mt2A were significantly lower in the PF and ZD groups than in the C group, and were significantly lower in the ZD group than in the PF group (Fig. 4). The mRNA expression level of HO-1 was not significantly different between the ZD group and the C and PF groups, but it was significantly higher in the PF group than in the C group. The mRNA expression level of Cu/Zn-SOD, which is one of the major families of superoxide dismutase that contains copper and zinc, was significantly lower in the ZD group than in the C and PF groups, with no difference between the C and PF groups. The mRNA expression levels of antioxidant enzymes, such as Mn-SOD, GR and GPx, were not significantly different between any of the groups.
Fig. 3.
Effect of dietary zinc deficiency on the gene expression levels of cytokines in femur. The mRNA expression levels of TNFα and IL1β were determined using qRT-PCR. The ordinate axis indicates the relative amount of mRNA compared with that from the C group. Gene expression levels were normalized to the levels of β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
Fig. 4.
Effect of dietary zinc deficiency on the mRNA expression levels of antioxidants and antioxidant enzymes in femur. The mRNA expression levels of Mt1a, Mt2A, HO-1, Cu/Zn-SOD, Mn-SOD, GR and GPx were determined using qRT-PCR. The ordinate axis indicates the relative amount of mRNA compared with that from the C group. Gene expression levels were normalized to the level of β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
Discussion
In Experiment I, we examined the effects of dietary zinc deficiency on oxidative stress in bone. Oxidative stress is a state of imbalance between the production of ROS and antioxidant mechanisms that leads to the oxidation of lipids, proteins, DNA and RNA molecules.(23) We found that dietary zinc deficiency increased the iron level in bone and a byproduct of lipid peroxidation, TBARS, which is considered a biomarker of the involvement of free radical damage in living organisms and is the most commonly applied marker for assaying lipid peroxidation in biomedical sciences. Iron is known to be involved in a series of single-electron transfer reactions that can generate ROS, which lead to tissue damage as a consequence of lipid, nucleic acid and/or protein oxidative damage.(15,16) In this study, we found a positive correlation between the TBARS level and the iron level in bone. However, measurements of the activities of several antioxidant enzymes, such as GPx and GR, and antioxidants, such as GSH, in bone showed no difference in these activities between any of the groups in this study. These results suggest that dietary zinc deficiency induces the accumulation of iron in bone, which is correlated with the TBARS level and is a factor that induces oxidative stress in bone.
In Experiment II, we attempted to clarify how oxidative stress affects abnormal bone development induced by zinc deficiency by examining the expression level of bone metabolism- and oxidative stress-related genes in bone. We demonstrated that the level of serum CTx, which is a product of type I collagen degradation during osteoclastic bone resorption, was higher in rats fed zinc-deficient diets than in rats fed control diets. These results are consistent with our previous study.(6) Osteoclastogenesis requires M-CSF and RANKL, which are expressed by osteoblasts. M-CSF binds to c-Fms, which stimulates osteoclast differentiation, function and survival.(24) Moreover, M-CSF has been demonstrated to stimulate the expression of RANK, which is the receptor of RANKL, in hematopoietic osteoclast precursor cells.(25) Binding of RANKL to RANK that is expressed on osteoclasts results in the recruitment of TRAF family adaptor proteins, such as TRAF2 and TRAF6, and activates c-Fos and NFATc1, which induces the expression of osteoclast-specific genes, such as TRAP and CTSK.(26) In the present study, we observed that the mRNA expression levels of M-CSF and c-Fms were higher in the ZD group than in the C and PF groups. In addition, we observed that the mRNA expression levels of osteoclastogenesis-related genes, including RANKL, TRAF6, c-Fos, NFATc1, TRAP and CTSK, were higher in the ZD group than in the C and PF groups. These results suggest that dietary zinc deficiency induced the expression of M-CSF and RANKL, which resulted in an increase in bone resorption.
Oxidative stress is known to be involved in osteoclastogenesis and bone resorption.(17) In this study using real-time PCR, we determined that the effects of dietary zinc deficiency on the expression of inflammatory cytokines genes, such as TNFα and IL1β, were closely related to oxidative stress.(27,28) We found that dietary zinc deficiency increased the mRNA expression levels of TNFα and IL1β in bone in this study. TNFα and IL1β are known to upregulate the expression of RANKL, which is a trigger that stimulates osteoclastic activity and controls bone regeneration and remodeling.(29,30) Therefore, an increase in oxidative stress might indirectly stimulate osteoclastic activity by augmenting the expression of inflammatory cytokines, such as TNFα and IL1β, which have been strongly implicated in bone loss due to dietary zinc deficiency. Furthermore, TNFα not only promotes osteoclast differentiation and activation together with RANKL, but it also has the potential to directly induce osteoclast differentiation in vitro.(31) Kanazawa and Kudo(32) have demonstrated that the cells from TRAF2-deficient mice showed severely reduced TNFα-induced osteoclastogenesis due to defects in TNFα-mediated activation of JNK, NF-κB and NFATc1, whereas TRAF2 overexpression induced the differentiation of osteoclast progenitors from wild-type mice into TRAP-positive multinucleated cells. In this study, we also observed that dietary zinc deficiency increased the mRNA expression of TRAF2 in bone. Thus, it appears that the osteoclastogenesis caused by dietary zinc deficiency may be stimulated by an elevated level of RANKL expression induced by an increase in TNFα and IL1β and results from the direct action of TNFα.
Oxidative stress results from a persistent imbalance between the production of highly ROS and antioxidant defenses. Excess ROS are immediately eliminated from the cell by the antioxidant defense mechanisms. In addition, it has been reported that antioxidant, such as carotenoids, suppresses osteoclast formation in vitro.(33) Therefore, in Experiment II, we measured the expression levels of several antioxidant enzymes, such as metallothionein, Cu/Zn-SOD, Mn-SOD, GPx and GR, in bone, although the activities of GPx and GR were not altered in rats fed zinc deficient diet. We observed that dietary zinc deficiency decreased the mRNA expression of Mt1a and Mt2A in bone. Mt1a and Mt2A are isoforms of metallothionein that are known to be regulated by cellular zinc concentrations and are involved in protecting cells from heavy metal toxicity and oxidative stress. Several studies have reported that dietary zinc deficiency decreased the expression levels of Mt1a and Mt2A in tissues, such as jejunum, colon, liver and kidney.(34,35) These results support our findings that the expression levels of Mt1a and Mt2A in bone were very sensitive to the intake of dietary zinc. Furthermore, metallothionein is also believed to be a scavenger of hydroxyl free radicals, which cause the oxidation of lipids, proteins and DNA, and oxidative stress is a potent inducer of metallothionein synthesis in many tissues, suggesting a direct role for it in antioxidant detoxification.(36) Therefore, a dramatic decrease in Mt1a and Mt2A gene expression may cause a further increase in oxidative stress in bone of rats fed a zinc-deficient diet. However, further studies are needed to clarify whether a decrease in Mt1a and Mt2A gene expression would directly affect lipid peroxidation induced by dietary zinc deficiency in bone.
The SOD family is the first defense against ROS as it catalyzes the dismutation of the superoxide anion into O2 and hydrogen peroxide. The hydrogen peroxide is then reduced to H2O by GPx. In this study, the mRNA levels of GPx and GR were not different between any of the groups; similar results were obtained for the activities of GPx and GR. In contrast, the mRNA level of Cu/Zn-SOD, which contains zinc and copper, was significantly lower in the ZD group than in the C and PF groups, while the mRNA level of Mn-SOD in bone was not different between any of the groups. Kawasaki et al.(37) reported that Cu/Zn-SOD activity was significantly lower in the bone marrow cells of rats fed zinc-deficient diets than in those of rats fed standard diets. These results suggested that dietary zinc deficiency increased the TBARS level through an increase in the biological action of superoxide radicals caused by a decrease in the mRNA expression of Cu/Zn-SOD in bone.
HO-1, formerly known as an antioxidant enzyme, is the rate-limiting enzyme that catalyzes the degradation of heme to produce biliverdin, iron and carbon monoxide; it is quickly up-regulated in cells and tissues in response to various oxidative stress.(38) We observed that the mRNA level of HO-1 was higher in the PF group than in the C group, although there was no significant difference in this level between the C and ZD groups. These observations suggested that the PF group, which was pair-fed to the ZD group, may have been under some kind of stress, conceivably caused by their limited diet. Recently, dietary zinc deficiency has been shown to impair the expression of HO-1, leading to an increase in oxidative damage in diabetic animals.(39) Moreover, a study has reported that zinc induced the expression and activity of Nrf2, which is known to regulate the expression of HO-1.(40) Therefore, we assumed that the PF group was under some kind of stress and that HO-1 was upregulated in response, whereas the ZD group was unable to respond in the same manner due to the zinc deficiency, and instead, there was an increase in TBARS level in bone. Interestingly, Ke et al.(41) demonstrated that a lack of HO-1 increased serum CTx and osteoclast numbers to stimulate RANKL signaling in mice. In addition, they reported that HO-1 decreased a sustained level of ROS in response to RANKL, as well as the number and activity of osteoclasts in vitro.(41) These results suggested that dietary zinc deficiency could not increase the HO-1 expression in response to oxidative stress in bone, which might lead to enhancement of the mRNA expression of osteoclastogenesis-related genes.
Zinc is well known to stimulate bone formation and mineralization, and zinc deficiency can cause growth retardation. We observed in this study that dietary zinc deficiency decreased the level of serum Osteocalcin, a marker of bone formation; this result is consistent with our previous study.(6) We also measured the gene expression levels of osteoblastogenesis-related factors as indicators of bone formation in this study. Similar to the result for serum Osteocalcin level, we observed that dietary zinc deficiency decreased the mRNA expression levels of BMP2, Rnux2, Osterix, Col1a1, ALP and Osteocalcin in bone. Runx2 is the major transcription factor controlling osteoblast commitment and differentiation. Osterix is a zinc finger transcription factor specifically expressed by osteoblasts that is important for osteoblast differentiation. Osterix acts downstream of Runx2, as it has been shown that Osterix-deficient mice expressed Runx2.(42) BMP2 target genes, including Runx2 and Osterix, are transcription factors that play essential roles in skeletal development.(43) ALP and Osteocalcin are markers of osteoblastogenesis; ALP is expressed in pre-osteoblasts and Osteocalcin is expressed in mature osteoblasts.(44) Therefore, the results of this study demonstrated that dietary zinc deficiency down-regulated the expression of osteoblast marker genes (ALP and Osteocalcin) through the down-regulation of osteoblast differentiation-related genes (Runx2 and Osterix) induced by a decrease in the mRNA level of BMP2. In addition, several studies have shown that ROS can affect the growth and function of osteoblast.(45,46) Arai et al.(47) have been reported that the gene expression levels of osteoblastogenesis-related factors, such as Runx2 and ALP was decreased in the H2O2 exposured cells. Therefore, these results suggested that oxidative stress caused by dietary zinc deficiency not only increase bone resorption but also decrease bone formation through the down-regulation of osteoblastgenesis-related genes.
In conclusion, we discovered that dietary zinc deficiency induced oxidative stress caused by iron accumulation and the down-regulation of antioxidants, such as Mt1a, Mt2A and Cu/Zn-SOD, in bone. We also demonstrated that dietary zinc deficiency enhanced the oxidative stress, and which induced cytokines-stimulated the expression of osteoclastogenesis-related genes, resulting in an increase in bone resorption. Furthermore, we clarified that dietary zinc deficiency decreased the expression of osteogenic genes, such as ALP, Osteocalcin, Col1a1, Runx2 and Osterix, via a decrease in the mRNA level of BMP2, resulting in a decrease in bone formation.
Acknowledgments
We thank Professor Yoshiko Ishimi for providing the machine that was used for the measurement of BA, BMC and BMD. We also thank Assistant Professor Tomoko Ishijima and Research Fellow Tsudoi Toyoda from the University of Tokyo for their assistance with the RNA extraction from bone. This work was supported by the Research Fund for the Advancement of the Graduate School, Tokyo University of Agriculture (research fund for Ph.D. candidates).
Abbreviations
- ALP
alkaline phosphatase
- BA
bone area
- BMC
bone mineral content
- BMD
bone mineral density
- BMP2
bone morphogenetic protein 2
- c-Fms
macrophage colony-stimulating factor receptor
- CTSK
cathepsin K
- CTx
C-terminal telopeptide of type I collagen
- Cu/Zn-SOD
CuZn-superoxide dismutase
- GSH
glutathione
- GPx
glutathione peroxidase
- GR
glutathione reductase
- HO-1
heme oxygenase 1
- IL1β
interleukin-1β
- M-CSF
macrophage colony-stimulating factor
- Mn-SOD
Mn-superoxide dismutase
- Mt1a
metallothionein 1
- Mt2A
metallothionein 2
- NFATc1
nuclear factor of activated T cells cytoplasmic 1
- OPG
osteoprotegerin
- RANKL
receptor for activator of NF-κB ligand
- Runx2
runt-related transcription factor 2
- TNFα
tumor necrosis factor-α
- TRAF2
tumor necrosis factor receptor-associated factor 2
- TRAF6
tumor necrosis factor receptor-associated factor 6
- TRAP
tartrate-resistant acid phosphatase
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata K, Enomoto H, Nishiguchi S, et al. Serum zinc value in patients with hepatitis virus-related chronic liver disease: association with the histological degree of liver fibrosis and with the severity of varices in compensated cirrhosis. J Clin Biochem Nutr. 2014;55:147–152. doi: 10.3164/jcbn.14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 4.Holloway WR, Collier FM, Herbst RE, Hodge JM, Nicholson GC. Osteoblast-mediated effects of zinc on isolated rat osteoclast: Inhibition of bone resorption and enhancement of osteoclast number. Bone. 1996;19:137–142. doi: 10.1016/8756-3282(96)00141-x. [DOI] [PubMed] [Google Scholar]
- 5.Togari A, Arakawa S, Arai M, Matsumoto S. Alteration of in vitro bone metabolism and tooth formation by zinc. Gen Pharmacol. 1993;24:1133–1140. doi: 10.1016/0306-3623(93)90360-a. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Kajita Y, Katsumata S, et al. Zinc deficiency increases serum concentrations of parathyroid hormone through a decrease in serum calcium and induces bone fragility in rats J Nutr Sci Vitaminol (Tokyo); in press. [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 8.Bao B, Prasad AS, Beck FWJ, et al. Zinc decreases C-reactive protein, lipid peroxidation, and implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaheen AA, el-Fattah AA. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int J Biochem Cell Biol. 1995;27:89–95. doi: 10.1016/1357-2725(94)00053-0. [DOI] [PubMed] [Google Scholar]
- 10.Gomez NN, Fernandez MR, Zirulnik F, et al. Chronic zinc deficiency induces an antioxidant adaptive response in rat lung. Exp Lung Res. 2003;29:485–502. doi: 10.1080/01902140303776. [DOI] [PubMed] [Google Scholar]
- 11.Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology. 2002;175:223–234. doi: 10.1016/s0300-483x(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 12.Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823–829. doi: 10.1093/jn/125.4.823. [DOI] [PubMed] [Google Scholar]
- 13.Keen CL, Golub MS, Gershwin ME, Lönnerdal B, Hurley LS. Studies of marginal zinc deprivation in rhesus monkeys. III. Use of liver biopsy in the assessment of zinc status. Am J Clin Nutr. 1988;47:1041–1045. doi: 10.1093/ajcn/47.6.1041. [DOI] [PubMed] [Google Scholar]
- 14.Rogers JM, Lönnerdal B, Hurley LS, Keen CL. Iron and zinc concentrations and 59Fe retention in developing fetuses of zinc-deficient rats. J Nutr. 1987;117:1875–1882. doi: 10.1093/jn/117.11.1875. [DOI] [PubMed] [Google Scholar]
- 15.Thomas CE, Morehouse LA, Aust SD. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985;260:3275–3280. [PubMed] [Google Scholar]
- 16.Carlin G, Djursäter R. Xanthine oxidase induced depolymerization of hyaluronic acid in the presence of ferritin. FEBS Lett. 1984;177:27–30. doi: 10.1016/0014-5793(84)80974-6. [DOI] [PubMed] [Google Scholar]
- 17.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Gimblet EG, Marney AF, Bonsnes RW. Determination of calcium and magnesium in serum, urine, diet, and stool by atomic absorption spectrophotometry. Clin Chem. 1967;13:204–214. [PubMed] [Google Scholar]
- 20.Gomori G. A modification of the colorimetric phosphorus determination for use with the photoelectric colorimeter. J Lab Clin Med. 1942;27:955–960. [Google Scholar]
- 21.Kikugawa K, Kojima T, Yamaki S, Kosugi H. Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylenediaminetetraacetic acid. Anal Biochem. 1992;202:249–255. doi: 10.1016/0003-2697(92)90102-d. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Arai F, Miyamoto T, Ohneda O, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappa B (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 28.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFα potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 30.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa K, Kudo A. TRAF2 is essential for TNF-α-induced osteoclastogenesis. J Bone Miner Res. 2005;20:840–847. doi: 10.1359/JBMR.041225. [DOI] [PubMed] [Google Scholar]
- 33.Tadaishi M, Nishide Y, Tousen Y, Kruger MC, Ishimi Y. Cooperative effects of soy isoflavones and carotenoids on osteoclast formation. J Clin Biochem Nutr. 2014;54:109–115. doi: 10.3164/jcbn.13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato M, Nagai Y. Effect of zinc deficiency on the accumulation of metallothionein and cadmium in the rat liver and kidney. Arch Environ Contam Toxicol. 1989;18:587–593. doi: 10.1007/BF01055026. [DOI] [PubMed] [Google Scholar]
- 35.Pfaffl MW, Windisch W. Influence of zinc deficiency on the mRNA expression of zinc transporters in adult rats. J Trace Elem Med Biol. 2003;17:97–106. doi: 10.1016/S0946-672X(03)80005-6. [DOI] [PubMed] [Google Scholar]
- 36.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki I, Suzuki Y, Yanagisawa H. Zinc deficiency enhances the induction of micronuclei and 8-hydroxy-2'-deoxyguanosine via superoxide radical in bone marrow of zinc-deficient rats. Biol Trace Elem Res. 2013;154:120–126. doi: 10.1007/s12011-013-9706-8. [DOI] [PubMed] [Google Scholar]
- 38.Maines MD. The heme oxygenase system: past, present, and future. Antioxid Redox Signal. 2004;6:797–801. doi: 10.1089/ars.2004.6.797. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Cui W, Tan Y, et al. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J Cell Mol Med. 2014;18:895–906. doi: 10.1111/jcmm.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortese MM, Suschek CV, Wetzel W, Kröncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Ke K, Safder MA, Sul OJ, et al. Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol Cell Endocrinol. 2015;409:11–20. doi: 10.1016/j.mce.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Hassan MQ, Tare RS, Lee SH, et al. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J Biol Chem. 2006;281:40515–40526. doi: 10.1074/jbc.M604508200. [DOI] [PubMed] [Google Scholar]
- 44.Javed A, Chen H, Ghori FY. Genetic and transcriptional control of bone formation. Oral Maxillofac Surg Clin North Am. 2010;22:283–293. doi: 10.1016/j.coms.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 46.Fatokun AA, Stone TW, Smith RA. Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: the effects of glutamate and protection by purines. See comment in PubMed Commons belowBone. 2006;39:542–551. doi: 10.1016/j.bone.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 47.Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]