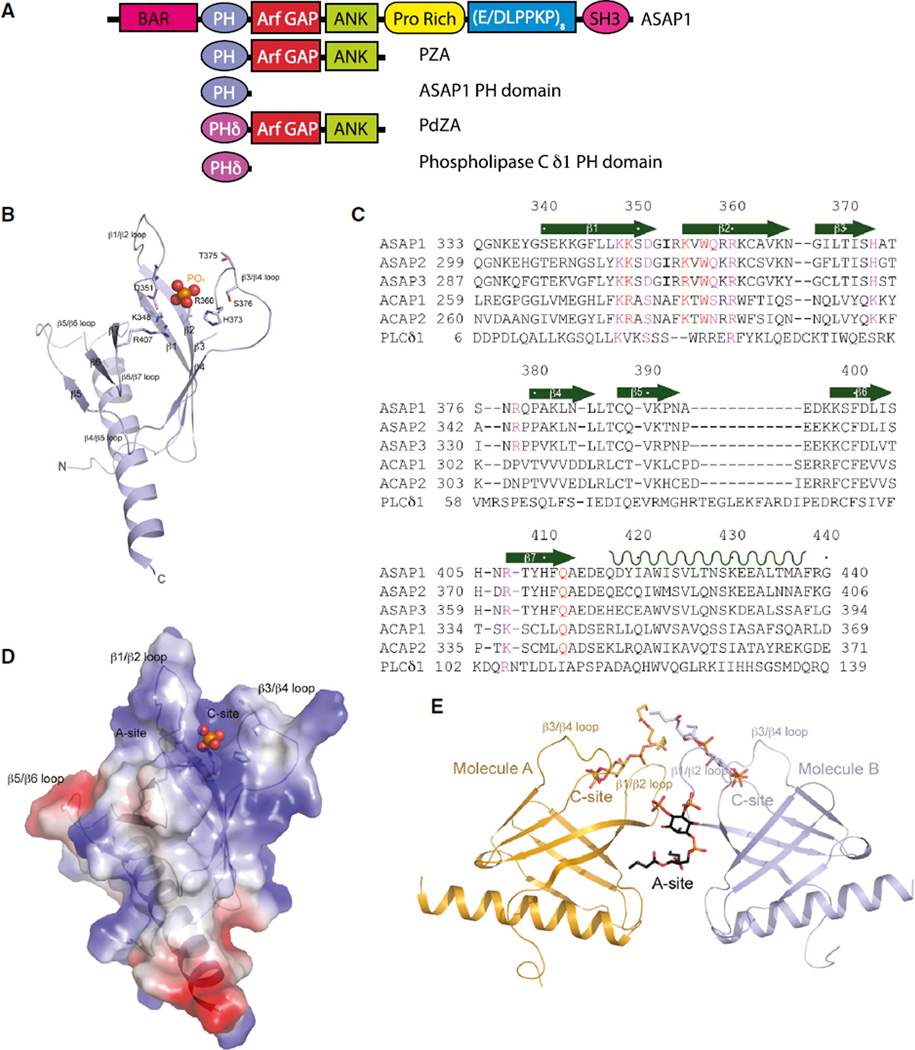
Figure 1. Structures of Unliganded PH Domain of ASAP1 and Its Complex with diC4-PtdIns(4,5)P2.
(A) Schematic representation of proteins used in this study.
(B) Ribbon representation of the structure of monomeric unliganded ASAP1 PH domain. Both N and C termini are labeled along with all β strands, as well as loops linking the β strands. The putative phosphate group is shown as a ball model with the phosphorus atom in orange and oxygen atoms in red. Residues that are interacting with the phosphate group are shown as stick models.
(C) Sequence of the ASAP1 PH domain aligned with other PH domains. Assignment of secondary structural elements defined by the crystal structure is shown. ASAP1, GI |4063616; ASAP2, GI |48474259; ASAP3, GI |221307473; ACAP1, GI |7661880; ACAP2, GI |39932727; PLCδ1, GI |130228.
(D) Electrostatic potential of monomeric ASAP1 PH domain. The surface representation of the electrostatic potential is superimposed on the ribbon structure of the ASAP1 PH domain. The phosphate group is shown as a ball model.
(E) One possible dimeric association of the two ASAP1 PH domains (A and B) in crystal. Ribbon representation of two molecules (A in coral and B in purple) in the asymmetric unit is shown. The two different PtdIns(4,5)P2 binding sites are illustrated as A site formed at the dimer interface and C site formed between β1/β2 and β3/β4 loops.
See also Figures S1, S2 and S3.