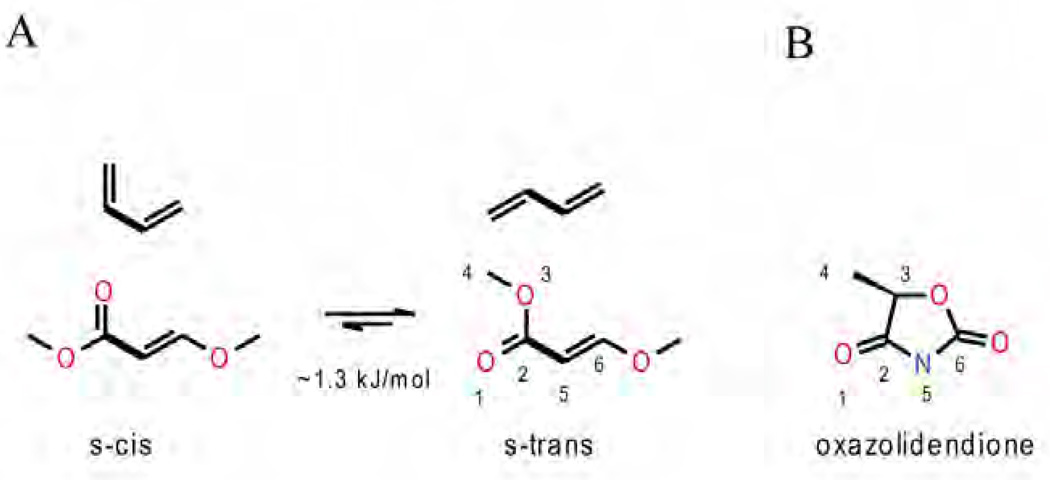
Figure 12. Chemical configurations of various toxophores.
(A) Illustration of s-cis and s-trans conformation of conjugated double bonds. (B) The oxazolidendione ring is oriented and its atoms are numbered (1–5) to optimally match the methoxy methyl acrylate (MOA) group to the left. The five-membered ring resembles a 'tied' back version of the MOA group.